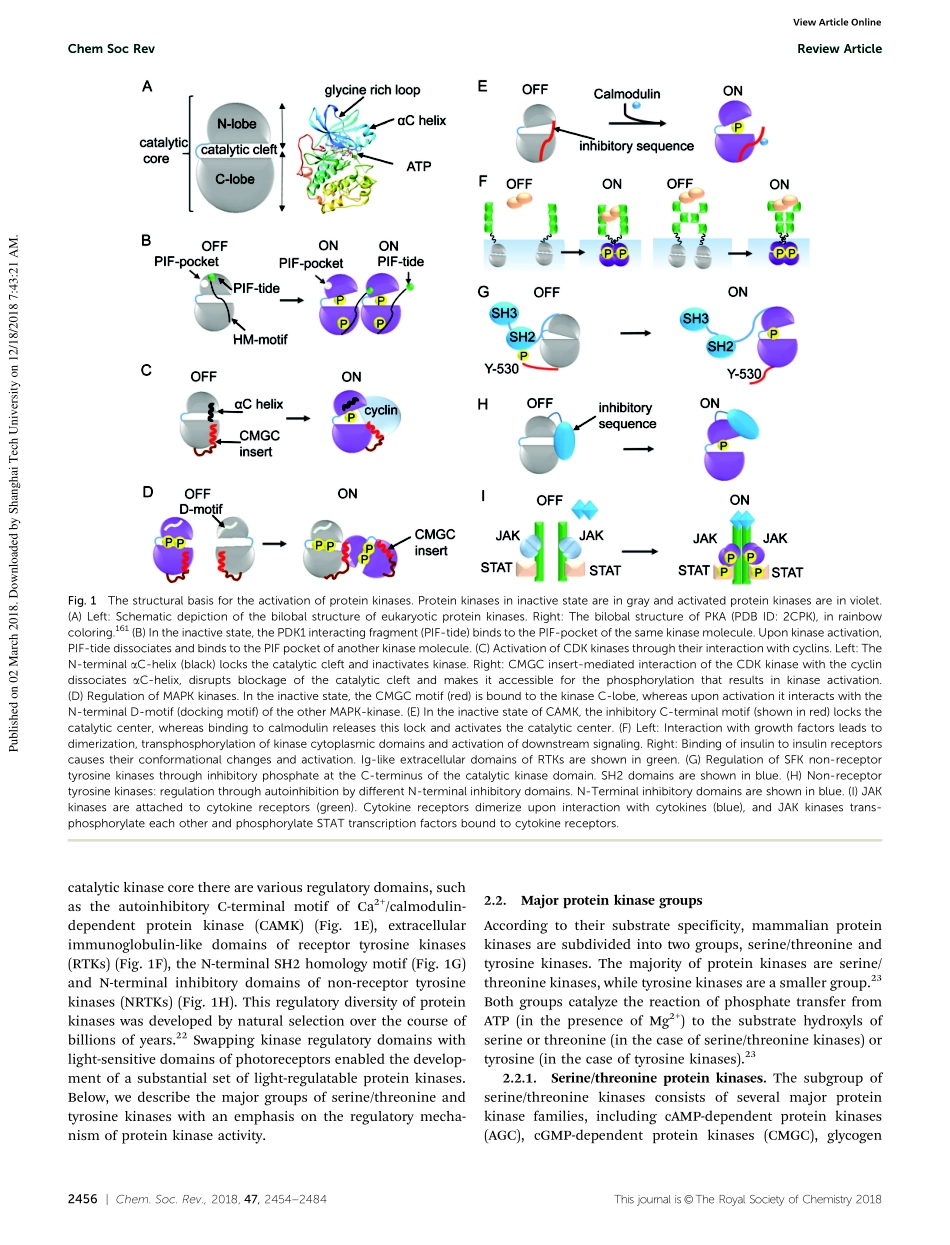
2454|Chem.Soc.Rev.,2018,47,2454--2484Thisjournalis©TheRoyalSocietyofChemistry2018Citethis:Chem.Soc.Rev.,2018,47,2454OptogeneticallycontrolledproteinkinasesforregulationofcellularsignalingAnnaV.Leopold,†aKonstantinG.Chernov†aandVladislavV.Verkhusha*abProteinkinasesareinvolvedintheregulationofmanycellularprocessesincludingcelldifferentiation,survival,migration,axonguidanceandneuronalplasticity.Agrowingsetofoptogenetictools,termedopto-kinases,allowsactivationandinhibitionofdifferentproteinkinaseswithlight.Theoptogeneticregulationenablesfast,reversibleandnon-invasivemanipulationofproteinkinaseactivities,complementingtraditionalmethods,suchastreatmentwithgrowthfactors,proteinkinaseinhibitorsorchemicaldimerizers.Inthisreview,wesummarizethepropertiesoftheexistingoptogenetictoolsforcontrollingtyrosinekinasesandserine–threoninekinases.Wediscusshowtheopto-kinasescanbeappliedforstudiesofspatialandtemporalaspectsofproteinkinasesignalingincellsandorganisms.Wecompareapproachesforchemicalandoptogeneticregulationofproteinkinaseactivityandpresentguidelinesforselectionofopto-kinasesandequipmenttocontrolthemwithlight.Wealsodescribestrategiestoengineernovelopto-kinasesonthebasisofvariousphotoreceptors.1.IntroductionReversiblephosphorylationisakeyregulatoryeventincellsignaling.Eukaryoticproteinsarephosphorylatedatserine,threonineandtyrosineresiduesbyproteinkinases.Phosphoryla-tiondownregulatesandupregulatesproteinactivity,tagsproteinsfordegradationandenablesthemtointeractwithotherproteins.1,2Proteinkinasescanbeclassifiedintotypicaleukaryoticproteinkinases(ePKs)thatshareasignificantstructuralsimilarityintheirproteinkinasedomains(Fig.1A–H)andatypicaleukaryoticaDepartmentofBiochemistryandDevelopmentalBiology,FacultyofMedicine,UniversityofHelsinki,Helsinki00290,FinlandbDepartmentofAnatomyandStructuralBiology,andGruss-LipperBiophotonicsCenter,AlbertEinsteinCollegeofMedicine,Bronx,NY10461,USA.E-mail:vladislav.verkhusha@einstein.yu.edu;Fax:+1-718-4308996;Tel:+1-718-4308591AnnaV.Leopold...