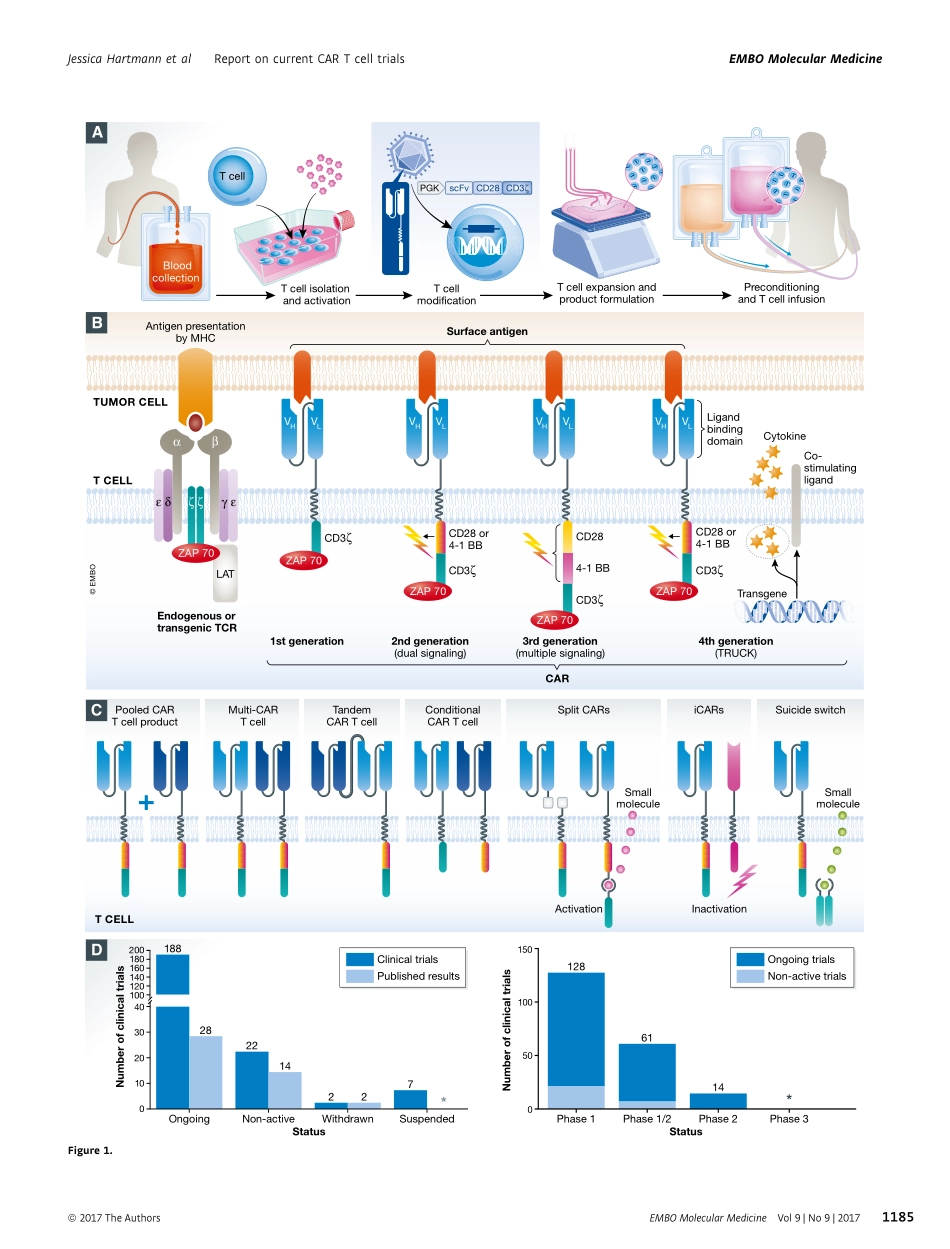
ReviewClinicaldevelopmentofCARTcells—challengesandopportunitiesintranslatinginnovativetreatmentconceptsJessicaHartmann1,*,MartinaSchüßler-Lenz2,3,AttilioBondanza4&ChristianJBuchholz1,3,**AbstractChimericantigenreceptor(CAR)Tcelltherapy,togetherwithcheckpointinhibition,hasbeencelebratedasabreakthroughtechnologyduetothesubstantialbenefitobservedinclinicaltrialswithpatientssufferingfromrelapsedorrefractoryB-cellmalignancies.Inthisreview,weprovideacomprehensiveover-viewoftheclinicaltrialsperformedsofarworldwideandanalyzeparameterssuchastargetedantigenandindication,CARmoleculardesign,CARTcellmanufacturing,anti-tumoractivities,andrelatedtoxicities.Morethan200CARTcellclinicaltrialshavebeeninitiatedsofar,mostofwhichaimtotreatlymphomaorleukemiapatientsusingCD19-specificCARs.Anincreasingnumberofstudiesaddresssolidtumorsaswell.Notably,notallclinicaltrialsconductedsofarhaveshownpromisingresults.Indeed,inafewpatientsCARTcelltherapyresultedinsevereadverseeventswithfataloutcome.Ofnote,lessthan10%oftheongoingCARTcellclinicaltrialsareperformedinEurope.Takingleadfromouranalysis,wediscusstheproblemsandgeneralhurdlespreventingefficientclinicaldevelopmentofCARTcellsaswellasopportunities,withaspecialfocusontheEuropeanstage.KeywordsATMPs;cancer;immunotherapy;regulatoryissues;toxicitiesDOI10.15252/emmm.201607485|Received19December2016|Revised3July2017|Accepted11July2017|Publishedonline1August2017EMBOMolMed(2017)9:1183–1197SeetheGlossaryforabbreviationsusedinthisarticle.IntroductionFormanydecades,cancertherapymainlyreliedonsurgery,chemotherapy,andradiotherapy.Inrecentyears,theconceptofstimulatingthepatient’simmuneresponseandtheobserveddura-bilityofresponseshasestablishedcancerimmunotherapiesasanoveltreatmentoptionforaseriesofcancertypes.Onepromis-ingapproachistheadoptivetransferofTcellsgeneticallyengi-neeredtoexpressachimericantigenreceptor(CAR)(Fig1A).SuchCARTcellsrecognizesurfaceantigensindependentlyfromMHCrestriction.Whentargetedtotumorsurfaceantigens,CARTcellsproliferateandkillt...