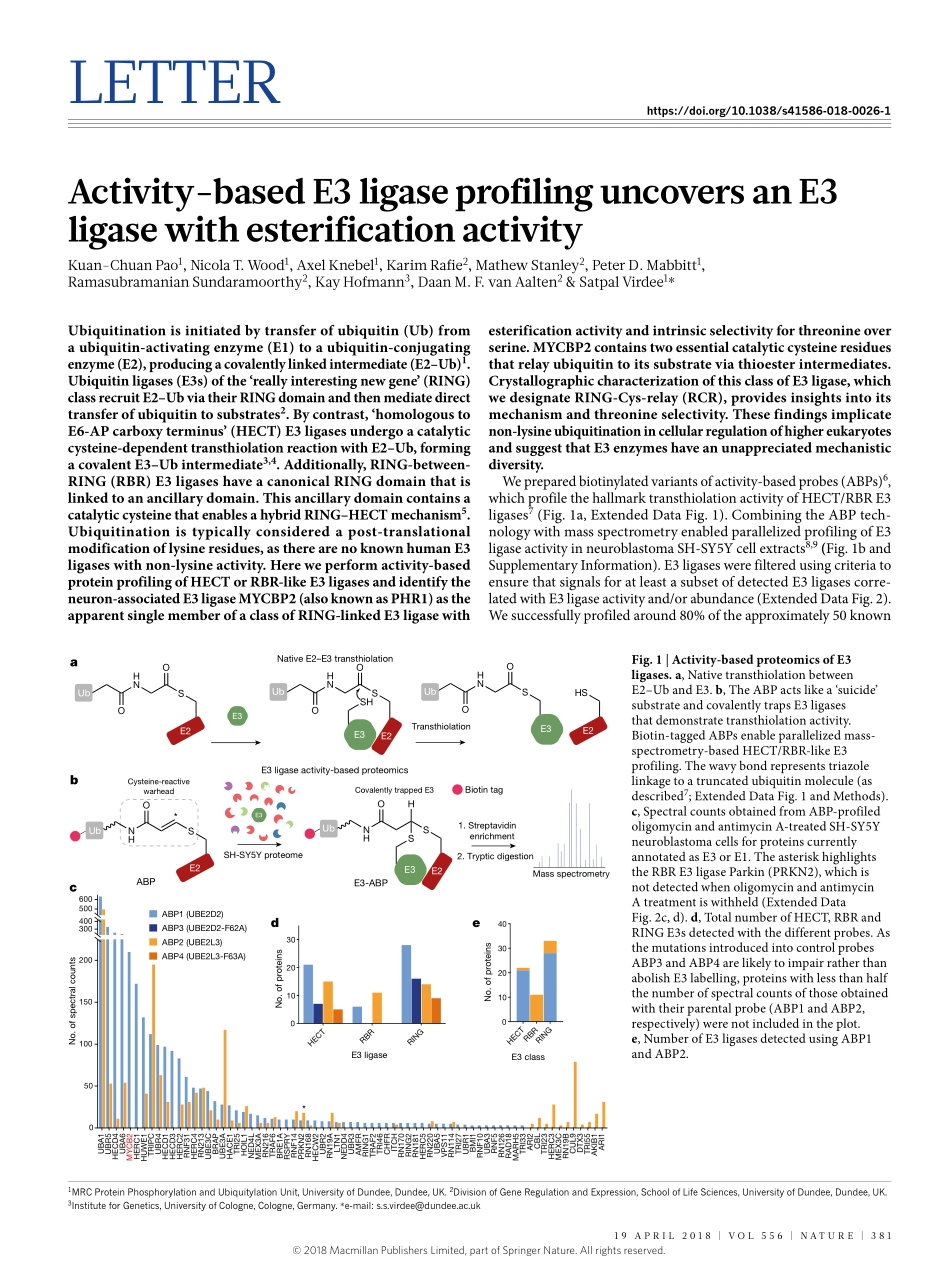
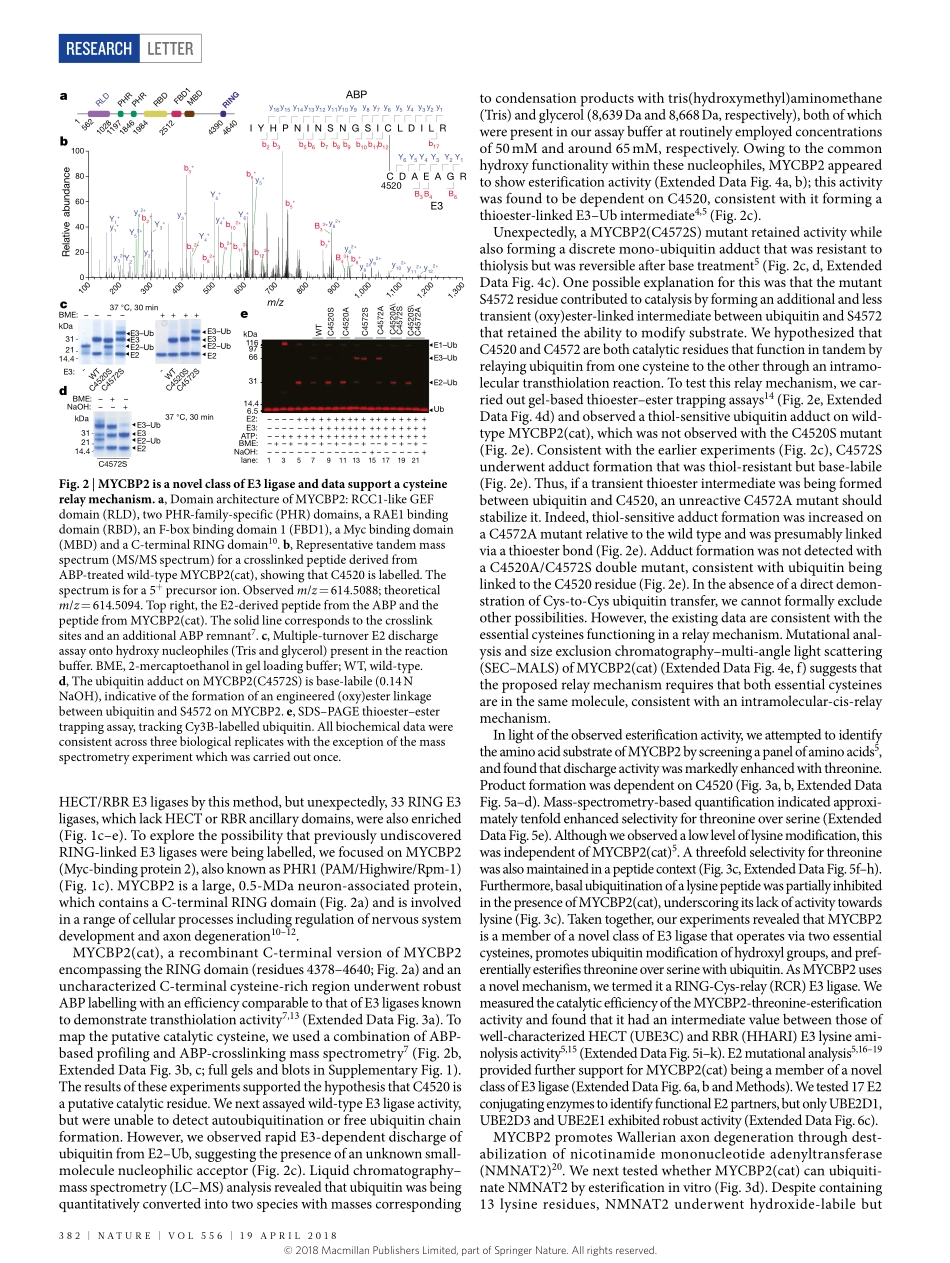
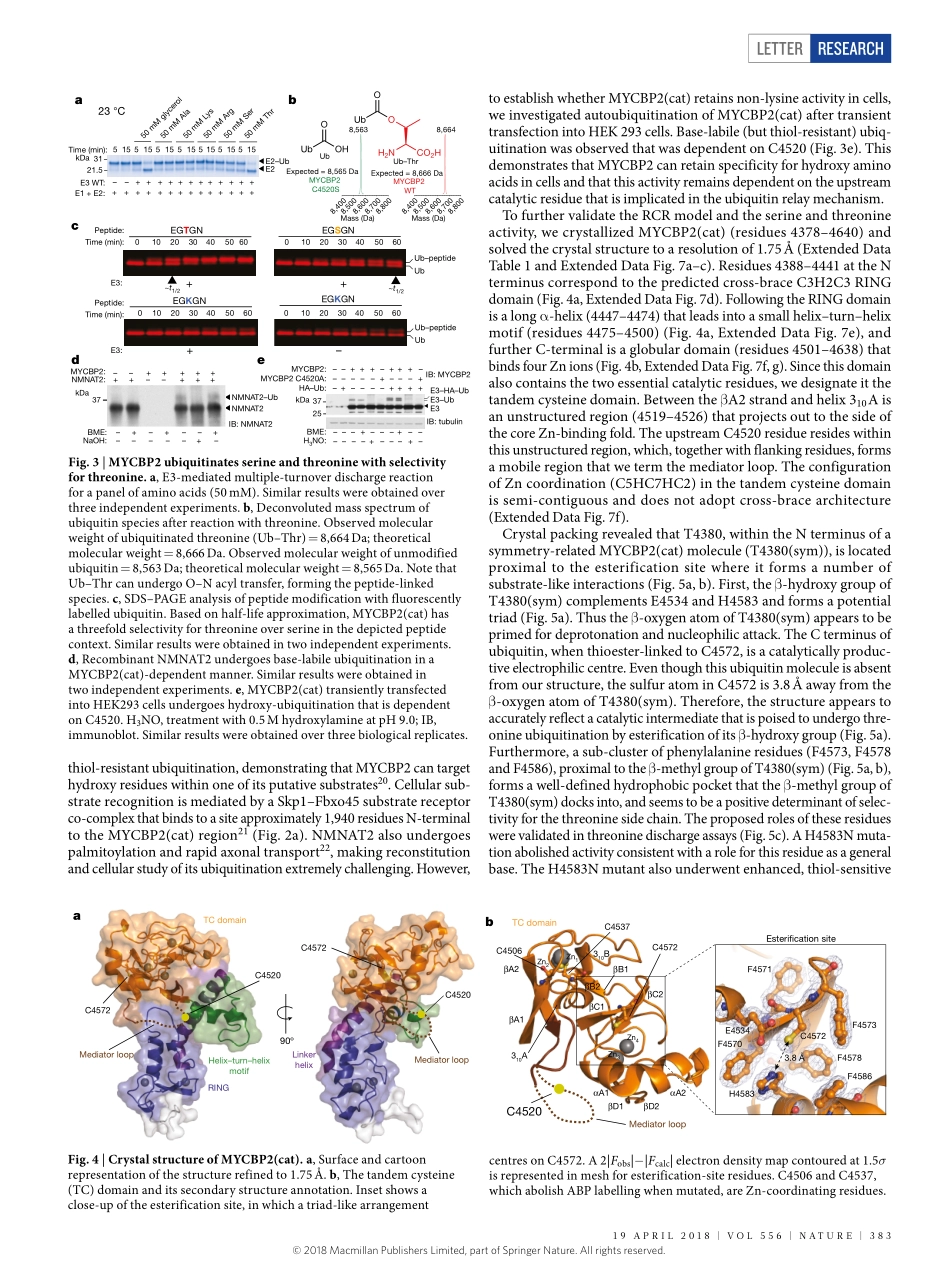
Letterhttps://doi.org/10.1038/s41586-018-0026-1Activity-basedE3ligaseprofilinguncoversanE3ligasewithesterificationactivityKuan-ChuanPao1,Nicolat.Wood1,AxelKnebel1,Karimrafie2,MathewStanley2,PeterD.Mabbitt1,ramasubramanianSundaramoorthy2,KayHofmann3,DaanM.F.vanAalten2&SatpalVirdee1*Ubiquitinationisinitiatedbytransferofubiquitin(Ub)fromaubiquitin-activatingenzyme(E1)toaubiquitin-conjugatingenzyme(E2),producingacovalentlylinkedintermediate(E2–Ub)1.Ubiquitinligases(E3s)ofthe‘reallyinterestingnewgene’(RING)classrecruitE2–UbviatheirRINGdomainandthenmediatedirecttransferofubiquitintosubstrates2.Bycontrast,‘homologoustoE6-APcarboxyterminus’(HECT)E3ligasesundergoacatalyticcysteine-dependenttransthiolationreactionwithE2–Ub,formingacovalentE3–Ubintermediate3,4.Additionally,RING-between-RING(RBR)E3ligaseshaveacanonicalRINGdomainthatislinkedtoanancillarydomain.ThisancillarydomaincontainsacatalyticcysteinethatenablesahybridRING–HECTmechanism5.Ubiquitinationistypicallyconsideredapost-translationalmodificationoflysineresidues,astherearenoknownhumanE3ligaseswithnon-lysineactivity.Hereweperformactivity-basedproteinprofilingofHECTorRBR-likeE3ligasesandidentifytheneuron-associatedE3ligaseMYCBP2(alsoknownasPHR1)astheapparentsinglememberofaclassofRING-linkedE3ligasewithesterificationactivityandintrinsicselectivityforthreonineoverserine.MYCBP2containstwoessentialcatalyticcysteineresiduesthatrelayubiquitintoitssubstrateviathioesterintermediates.CrystallographiccharacterizationofthisclassofE3ligase,whichwedesignateRING-Cys-relay(RCR),providesinsightsintoitsmechanismandthreonineselectivity.Thesefindingsimplicatenon-lysineubiquitinationincellularregulationofhighereukaryotesandsuggestthatE3enzymeshaveanunappreciatedmechanisticdiversity.Wepreparedbiotinylatedvariantsofactivity-basedprobes(ABPs)6,whichprofilethehallmarktransthiolationactivityofHECT/RBRE3ligases7(Fig.1a,ExtendedDataFig.1).CombiningtheABPtech-nologywithmassspectrometryenabledparallelizedprofilingofE3ligaseactivityinneu...