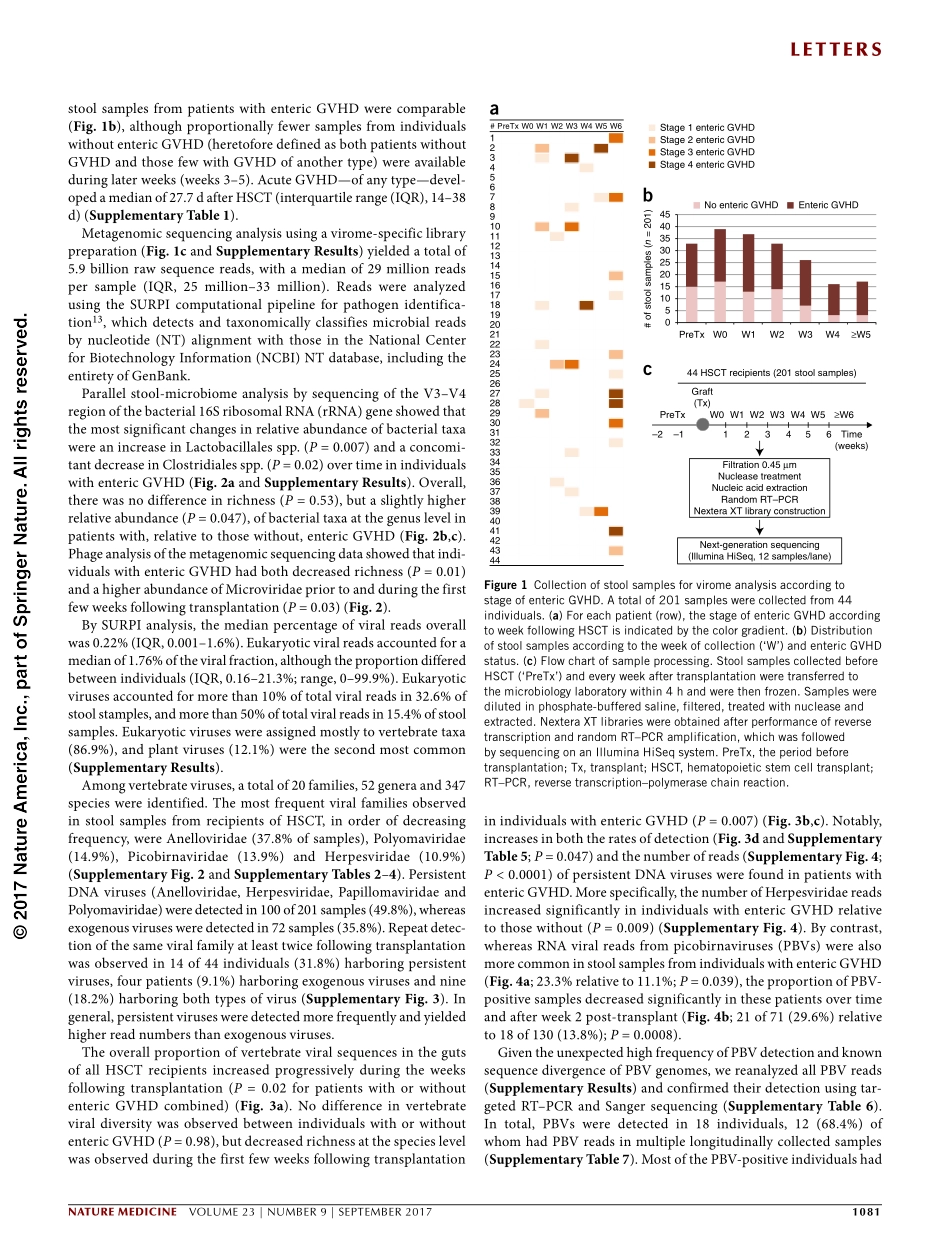
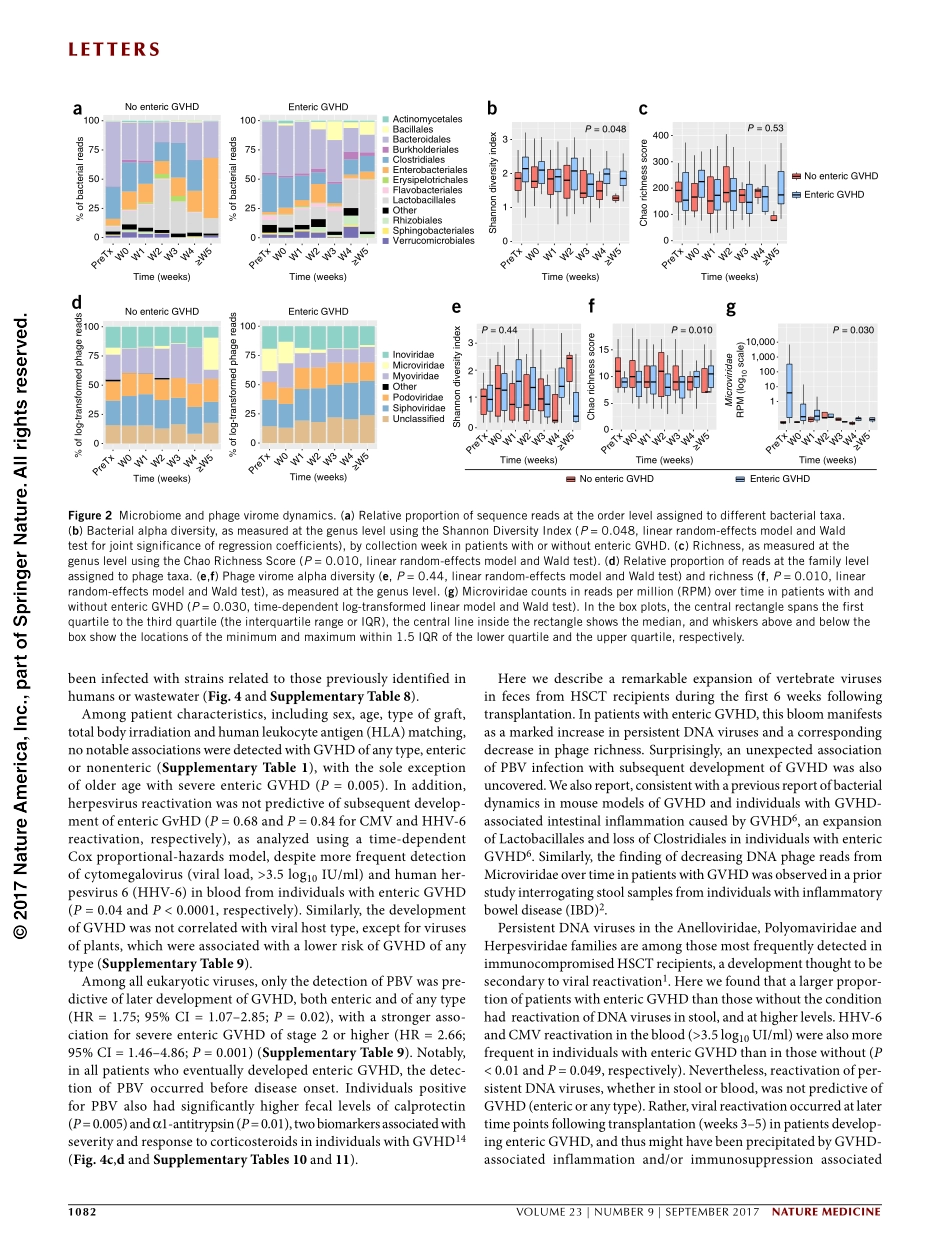
letters1080VOLUME23|NUMBER9|SEPTEMBER2017naturemedicineMuchattentionhasbeenfocusedontheroleofthebacterialmicrobiomeinhumanhealth,buttheviromeisunderstudied.Althoughpreviouslyinvestigatedinindividualswithinflammatoryboweldiseasesorsolid-organtransplants1,2,viromedynamicsinallogeneichematopoieticstemcelltransplantation(HSCT)andentericgraft-versus-hostdisease(GVHD)remainunexplored.Herewecharacterizethelongitudinalgutviromein44recipientsofHSCTusingmetagenomics.Aviral‘bloom’wasidentified,andsignificantincreasesweredemonstratedintheoverallproportionofvertebrateviralsequencesfollowingtransplantation(P=0.02).Increasesinboththeratesofdetection(P<0.0001)andnumberofsequences(P=0.047)ofpersistentDNAviruses(anelloviruses,herpesviruses,papillomavirusesandpolyomaviruses)overtimewereobservedinindividualswithentericGVHDrelativetothosewithout,afindingaccompaniedbyareducedphagerichness(P=0.01).Picobirnavirusesweredetectedin18individuals(40.9%),morefrequentlybeforeorwithinaweekaftertransplantthanatlatertimepoints(P=0.008).Inatime-dependentCoxproportional-hazardsmodel,picobirnaviruseswerepredictiveoftheoccurrenceofsevereentericGVHD(hazardratio,2.66;95%confidenceinterval(CI)=1.46–4.86;P=0.001),andcorrelatedwithhigherfecallevelsoftwoGVHDseveritymarkers,calprotectinanda1-antitrypsin.TheseresultsrevealaprogressiveexpansionofvertebrateviralinfectionsovertimefollowingHSCT,andtheysuggestanunexpectedassociationofpicobirnaviruseswithearlypost-transplantGVHD.Viruses,arisingmainlyfromtheHerpesviridaeandAdenoviridaefamilies,havebeenreportedtobefrequentlylinkedtoentericGVHD3–5.ThesevirusesareassociatedwithlatentinfectionsthatcanbereactivatedinthesettingofhostimmunosuppressionfromHSCT.Activeviralreplicationfromreactivation,resultingindynamicshiftsingutviralloads,maytriggerintestinalinflammationanddysregula-tionoftherestoredimmuneresponse,whichthenleadstotissuedam-ageandenhancedriskofGVHD.HSCTinducesprofoundchangesinbacterialmicrobiotadiversityandmaypotentiallyimpactthegutviromeinaparallelfashi...