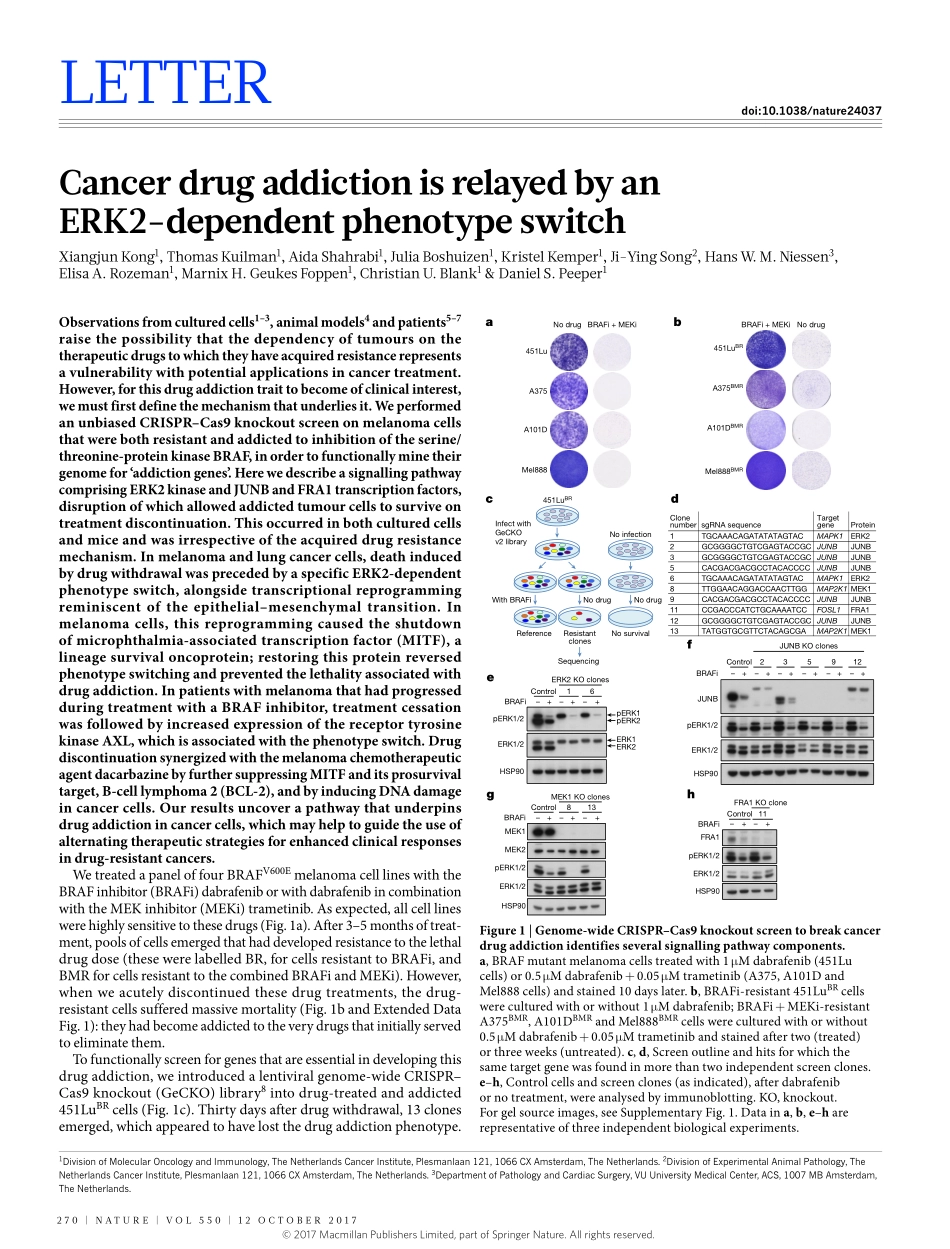
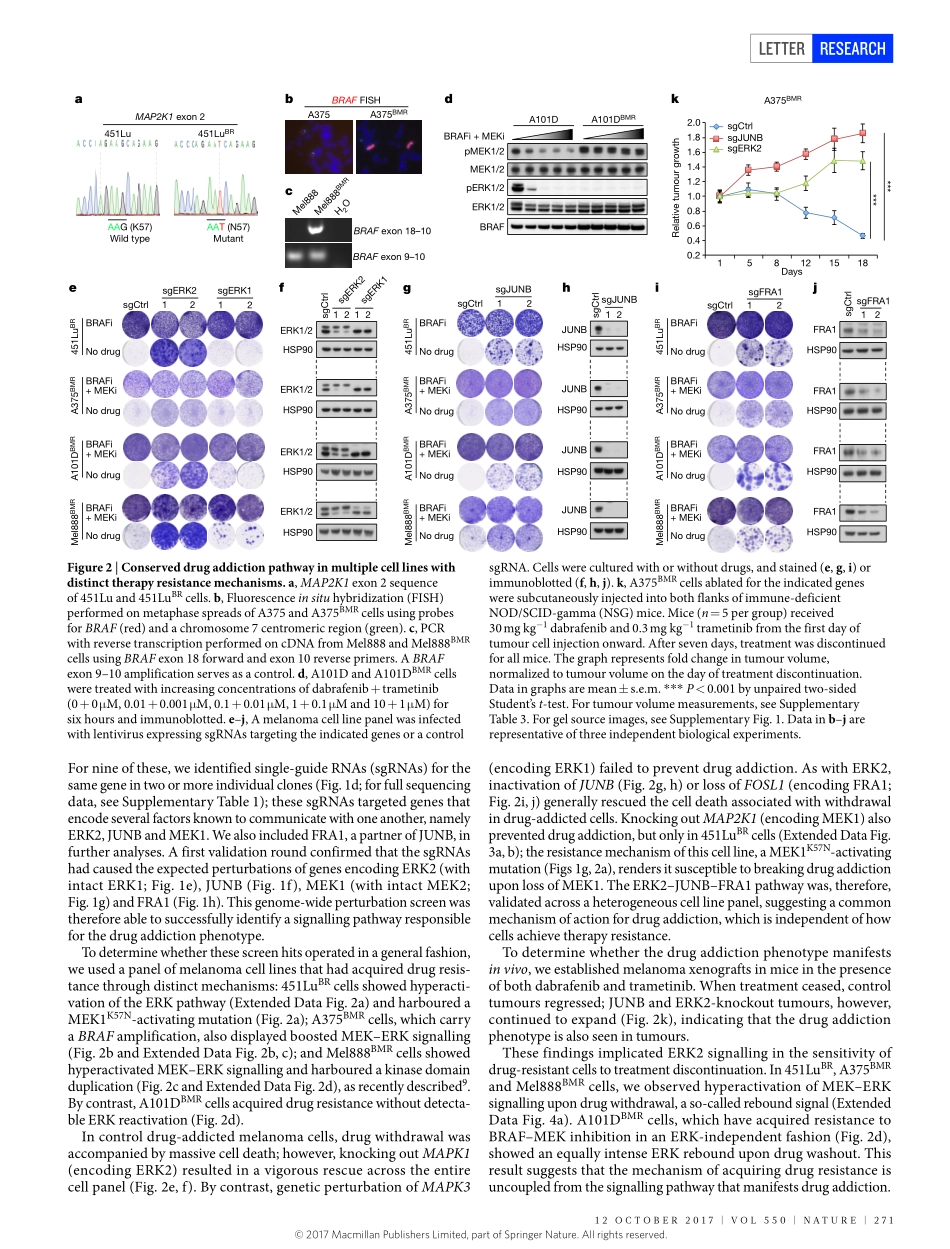
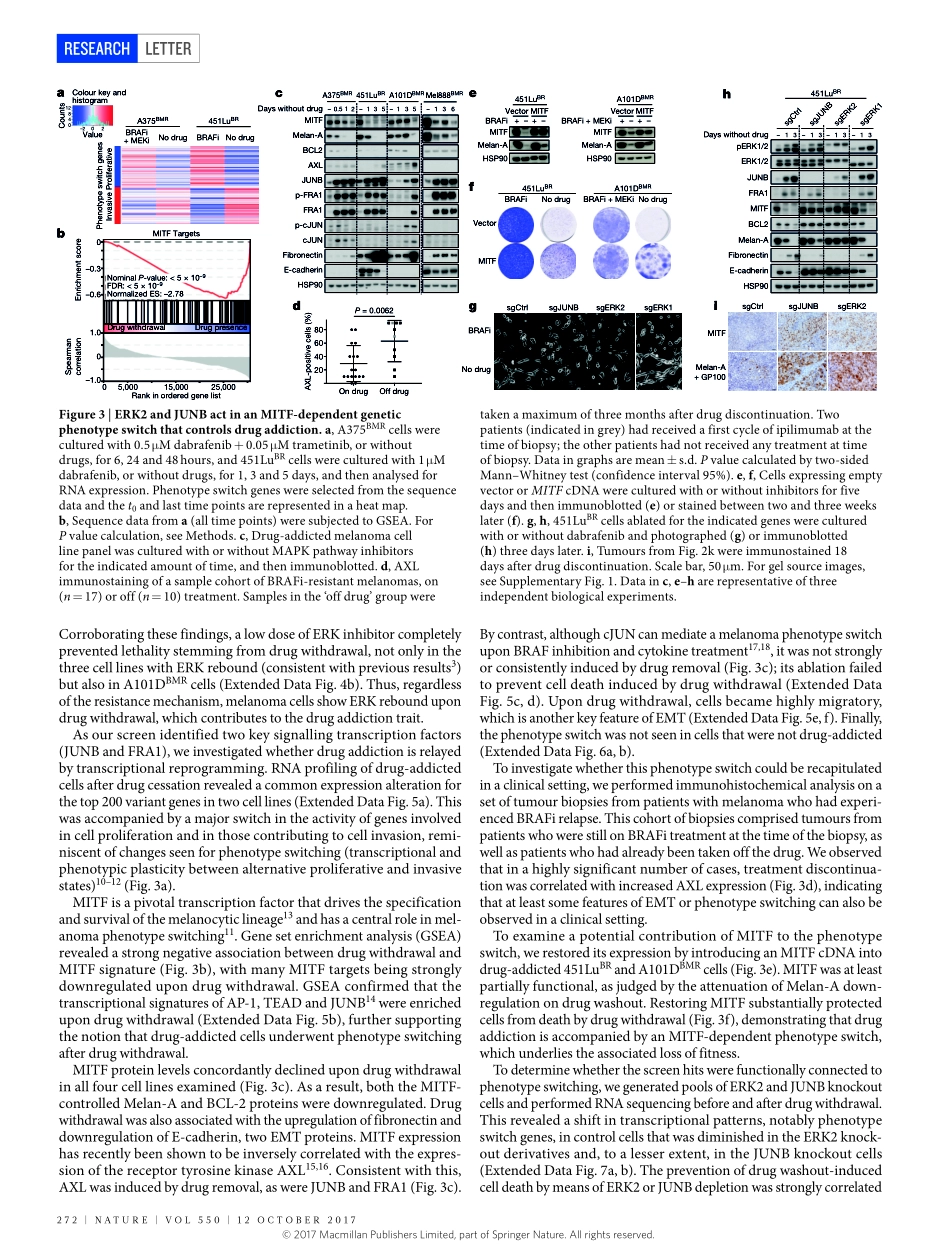
270|NATURE|VOL550|12OcTObER2017LETTERdoi:10.1038/nature24037CancerdrugaddictionisrelayedbyanERK2-dependentphenotypeswitchXiangjunKong1,ThomasKuilman1,AidaShahrabi1,Juliaboshuizen1,KristelKemper1,Ji-YingSong2,HansW.M.Niessen3,ElisaA.Rozeman1,MarnixH.GeukesFoppen1,christianU.blank1&DanielS.Peeper1Observationsfromculturedcells1–3,animalmodels4andpatients5–7raisethepossibilitythatthedependencyoftumoursonthetherapeuticdrugstowhichtheyhaveacquiredresistancerepresentsavulnerabilitywithpotentialapplicationsincancertreatment.However,forthisdrugaddictiontraittobecomeofclinicalinterest,wemustfirstdefinethemechanismthatunderliesit.WeperformedanunbiasedCRISPR–Cas9knockoutscreenonmelanomacellsthatwerebothresistantandaddictedtoinhibitionoftheserine/threonine-proteinkinaseBRAF,inordertofunctionallyminetheirgenomefor‘addictiongenes’.HerewedescribeasignallingpathwaycomprisingERK2kinaseandJUNBandFRA1transcriptionfactors,disruptionofwhichallowedaddictedtumourcellstosurviveontreatmentdiscontinuation.Thisoccurredinbothculturedcellsandmiceandwasirrespectiveoftheacquireddrugresistancemechanism.Inmelanomaandlungcancercells,deathinducedbydrugwithdrawalwasprecededbyaspecificERK2-dependentphenotypeswitch,alongsidetranscriptionalreprogrammingreminiscentoftheepithelial–mesenchymaltransition.Inmelanomacells,thisreprogrammingcausedtheshutdownofmicrophthalmia-associatedtranscriptionfactor(MITF),alineagesurvivaloncoprotein;restoringthisproteinreversedphenotypeswitchingandpreventedthelethalityassociatedwithdrugaddiction.InpatientswithmelanomathathadprogressedduringtreatmentwithaBRAFinhibitor,treatmentcessationwasfollowedbyincreasedexpressionofthereceptortyrosinekinaseAXL,whichisassociatedwiththephenotypeswitch.DrugdiscontinuationsynergizedwiththemelanomachemotherapeuticagentdacarbazinebyfurthersuppressingMITFanditsprosurvivaltarget,B-celllymphoma2(BCL-2),andbyinducingDNAdamageincancercells.Ourresultsuncoverapathwaythatunderpinsdrugaddictionincancercells,whichmayhelptoguidetheuseofalternating...