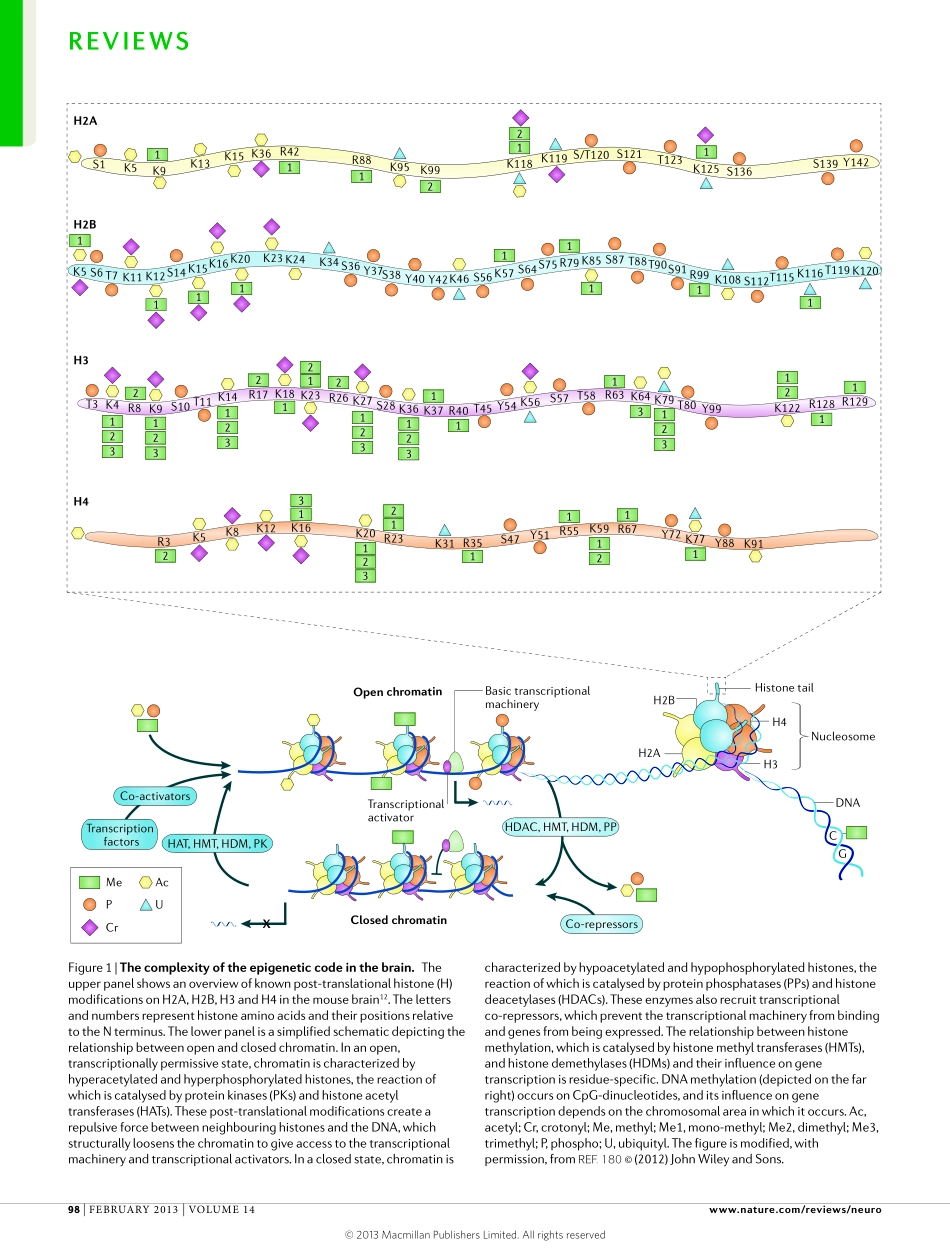
Bydefinition,amnemonicisadevicethatisintendedtoassistmemory.Ashumanmemoriesoutlastthehalf-lifeofmostbiologicalmolecules,anymolecularmnemonic—ifthereisone—shouldbesituatedonbiologicallyendur-ingmaterial.OnesuchmoleculeisDNA,whichcarriesinformationacrossgenerations.In1984,FrancisCrick(1916–2004)speculatedthat“memorymightbecodedinalterationstoparticularstretchesofchromosomalDNA”1.AlthoughCrickhimselfqualifiedhisspeculationas“notverylikely”,wenowknowthatchromatin,thecarrierofchromosomalDNA,canassistinstoringmemory-relatedinformationbyepigeneticmodifications2.Epigeneticmodificationsaredefinedas“thestructuraladaptationofchromosomalregionssoastoregister,signal,orperpetu-atealteredactivitystates”3.Bythisdefinition,epigeneticmodificationsfulfiltwofundamentalcharacteristicsofamnemonic:theyreacttolearning(thatis,neuronalactivitytriggeredbynewinformation);andtheycanconveysuchinformationintospecificgeneexpressionprogrammes,whichareaprerequisiteforlong-lastingmemories4.Oftheseveraltypesofepigeneticmodificationsthathavebeenassociatedwithcognitivefunctions—includingpost-translationalmodificationofhistoneproteinsbyacetylation,methylation,phosphorylation,DNAmethylationandRNAi5—histoneacetylationismostrobustlyassociatedwithpromotingmemoryfor-mation.Here,wereviewthegrowingbodyofevidencethathistoneacetylationmarksfunctionasmolecularmnemonics;describetheimplicationofincreasedanddecreasedhistoneacetylationinenhancingandconstrainingcognitivefunctions,respectively;anddiscussthepotentialofhistonedeacetylaseinhibitors(HDACis)astherapeuticagentsagainstcognitiveimpairmentsinseveralneurologicaldisorders.HistoneacetylationasmemoryaidsTheroleofhistoneacetylation.Inhistoneacetylation,anegativelychargedacetylgroupisaddedtolysineresiduesonhistoneproteins.Histoneacetylationisregulatedbytheopposingactionofhistoneacetyltransferases(HATs)andHDACs.TheadditionofacetylgroupsiscatalysedbyHATs,whicharedividedintotheGNAT,MYSTandp300/CBPsubfamilies6,whereastheremovalofacetylgroupsiscatalysedbyHDAC...