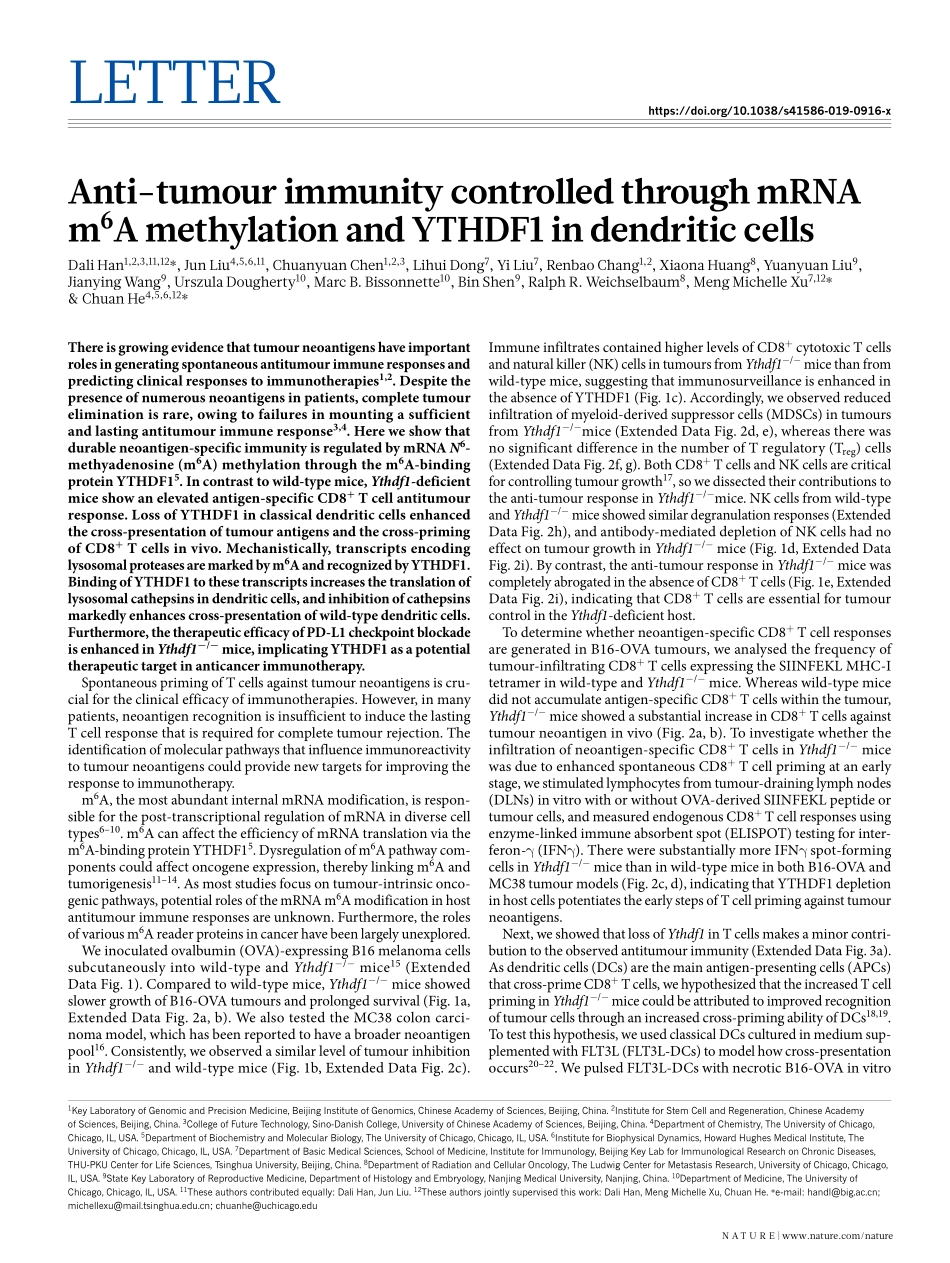
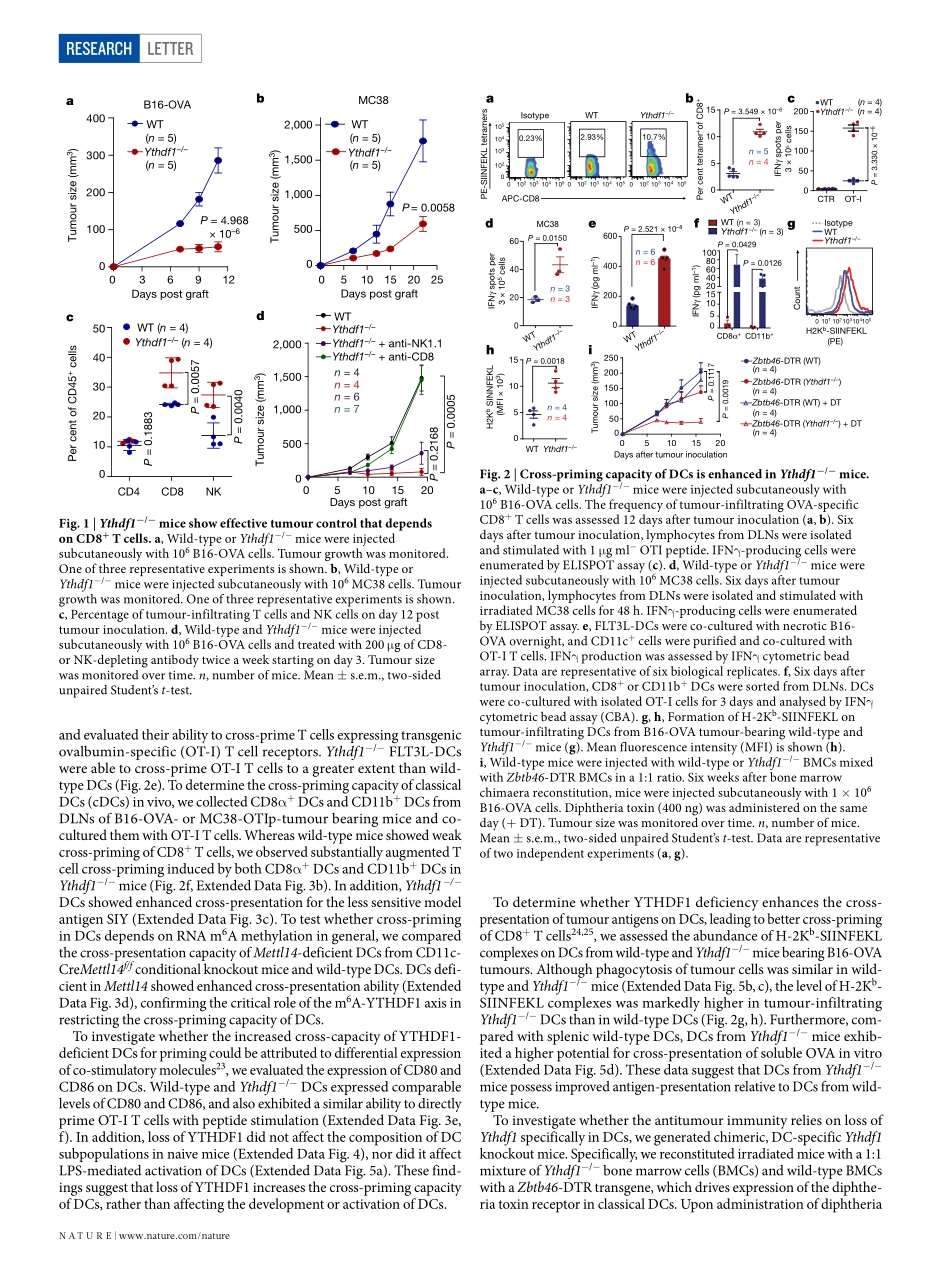
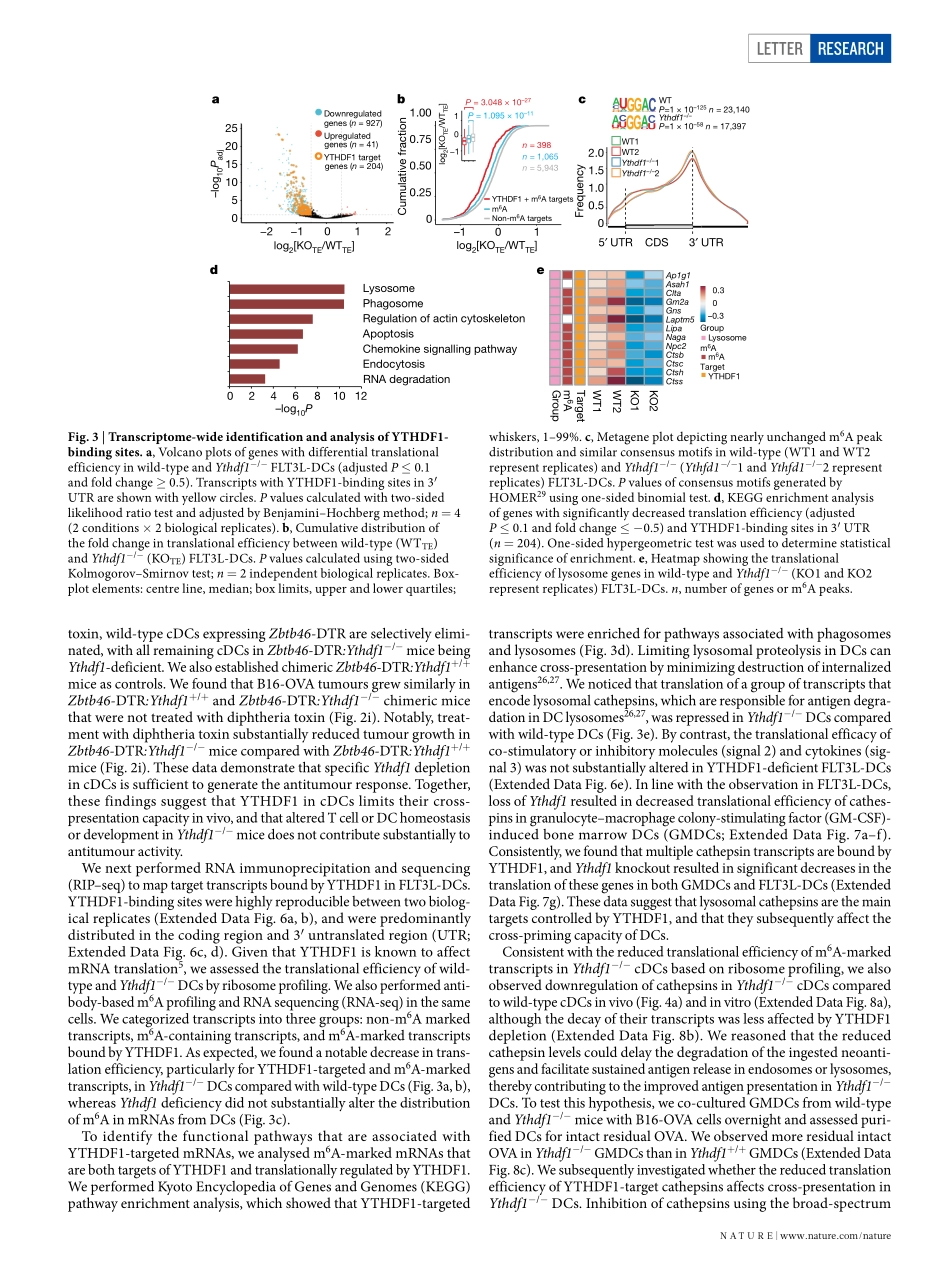
Letterhttps://doi.org/10.1038/s41586-019-0916-xAnti-tumourimmunitycontrolledthroughmRNAm6AmethylationandYTHDF1indendriticcellsDaliHan1,2,3,11,12*,JunLiu4,5,6,11,ChuanyuanChen1,2,3,LihuiDong7,YiLiu7,renbaoChang1,2,XiaonaHuang8,YuanyuanLiu9,JianyingWang9,UrszulaDougherty10,MarcB.Bissonnette10,BinShen9,ralphr.Weichselbaum8,MengMichelleXu7,12*&ChuanHe4,5,6,12*Thereisgrowingevidencethattumourneoantigenshaveimportantrolesingeneratingspontaneousantitumourimmuneresponsesandpredictingclinicalresponsestoimmunotherapies1,2.Despitethepresenceofnumerousneoantigensinpatients,completetumoureliminationisrare,owingtofailuresinmountingasufficientandlastingantitumourimmuneresponse3,4.Hereweshowthatdurableneoantigen-specificimmunityisregulatedbymRNAN6-methyadenosine(m6A)methylationthroughthem6A-bindingproteinYTHDF15.Incontrasttowild-typemice,Ythdf1-deficientmiceshowanelevatedantigen-specificCD8+Tcellantitumourresponse.LossofYTHDF1inclassicaldendriticcellsenhancedthecross-presentationoftumourantigensandthecross-primingofCD8+Tcellsinvivo.Mechanistically,transcriptsencodinglysosomalproteasesaremarkedbym6AandrecognizedbyYTHDF1.BindingofYTHDF1tothesetranscriptsincreasesthetranslationoflysosomalcathepsinsindendriticcells,andinhibitionofcathepsinsmarkedlyenhancescross-presentationofwild-typedendriticcells.Furthermore,thetherapeuticefficacyofPD-L1checkpointblockadeisenhancedinYthdf1−/−mice,implicatingYTHDF1asapotentialtherapeutictargetinanticancerimmunotherapy.SpontaneousprimingofTcellsagainsttumourneoantigensiscru-cialfortheclinicalefficacyofimmunotherapies.However,inmanypatients,neoantigenrecognitionisinsufficienttoinducethelastingTcellresponsethatisrequiredforcompletetumourrejection.Theidentificationofmolecularpathwaysthatinfluenceimmunoreactivitytotumourneoantigenscouldprovidenewtargetsforimprovingtheresponsetoimmunotherapy.m6A,themostabundantinternalmRNAmodification,isrespon-sibleforthepost-transcriptionalregulationofmRNAindiversecelltypes6–10.m6AcanaffecttheefficiencyofmRNAtranslat...