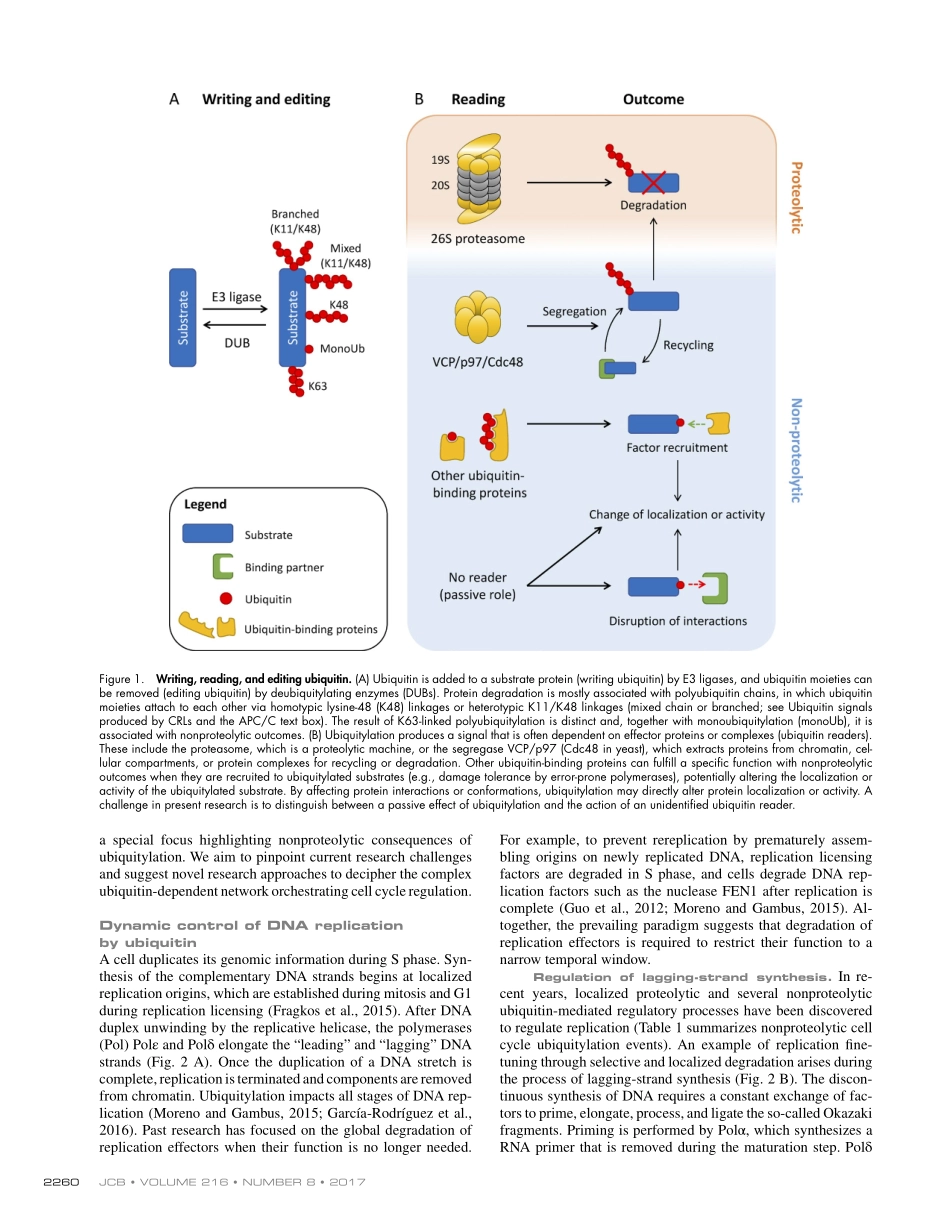
JCBJCB:ReviewTHEJOURNALOFCELLBIOLOGY2259TheRockefellerUniversityPressJ.CellBiol.Vol.216No.82259–2271https://doi.org/10.1083/jcb.201703170IntroductionCellproliferationisacontinuouscycleofDNAsynthesisandsubsequentchromosomeseparation.Posttranslationalmod-ificationsofeffectorproteinsensurethatthesemajoreventsandtheirtransitionsareorchestratedsothatgenomicinforma-tionispreserved.Thecovalentconjugationofthesmallpro-teinubiquitinthroughaprocesscalledubiquitylationplaysacriticalroleintheoverallregulationofcelldivision.Itiswellestablishedthatubiquitylationisasignalforproteindegrada-tionbytheproteasome(Fig.1,AandB),withspecialimpor-tanceinassuringorderedandwell-timedcellcycletransitions(TeixeiraandReed,2013;Bassermannetal.,2014).However,ubiquitylationisnotnecessarilylinkedtoproteindegrada-tion,andinrecentyears,anincreasingnumberofnonproteo-lyticoutcomesofproteinubiquitylationhavebeenreportedtoplayimportantcellularroles(KomanderandRape,2012).Proteasome-independentregulationofanubiquitylationtargetisachievedbychangesinprotein–proteininteractions,subcel-lularlocalization,orenzymeactivity(Fig.1B).Asopposedtotheirreversiblefateofdegradation,nonproteolyticoutcomesofubiquitylationallowforfunctionalfine-tuning,dynamicallyandreversiblyrespondingtointracellularcuesinsteadofrequiringdenovoproteinsynthesis.UbiquitinconjugationtoitstargetsrequirestheconcertedactionofanE1ubiquitin-activatingenzyme,E2ubiquitin-conjugatingenzyme,andE3ubiquitinligase.Thelatterbindsspecificallytothesubstrateandpromotesthetransferofubiqui-tintooneofitslysineresidues(seetextboxforanoverviewofE3ligasesinvolvedincellcycleregulation).Becauseofmulti-plereactivesitesonubiquitin,moremoietiesmaybeadded,es-tablishingcomplexoligomersorchains(Fig.1A).Thisenablesthatmultipleubiquitintopologiesgenerateindividualsignals,whicharecollectivelyreferredtoastheubiquitincode(Koman-derandRape,2012).Thiscodeisreadbydownstreamfactorscontainingubiquitin-bindingdomains,referredtoasreadersordecoders,whichspecificallyrecognizethecha...