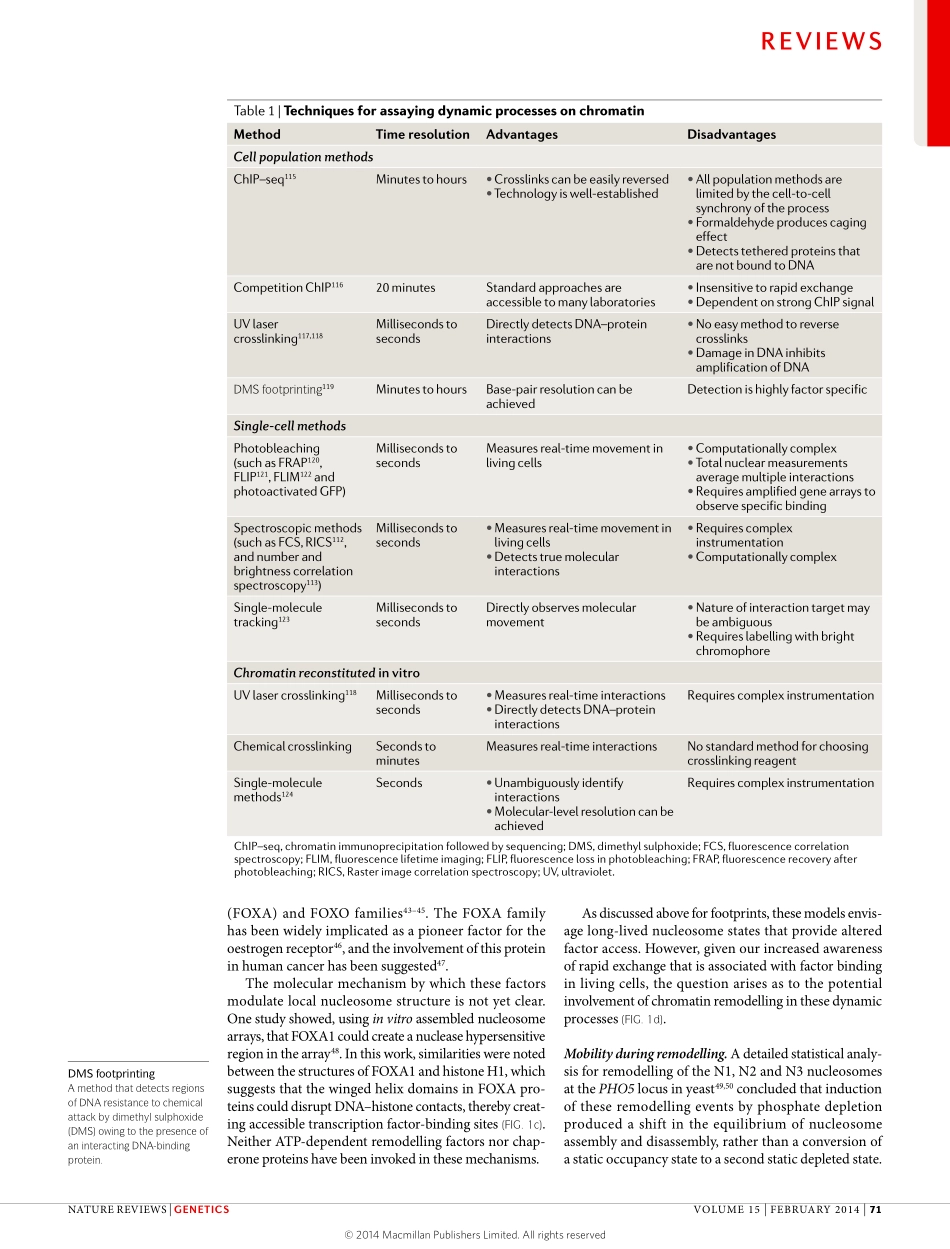
Theprocessesofdevelopmentanddifferentiationineukaryoticsystemsareregulatedbyconstantlychang-ingcohortsofsite-specificDNA-bindingproteinsthatdirectcell-selectivetranscriptionalprogrammes.Acen-tralparadigmincurrentbiologyarguesthatthesefactorsinteractwithregulatoryelements(thatis,enhancers)togoverntheactivitystatesoftargetpromotersinacell-selectivemanner.Inbacterialsystems,site-specificrecognitionofDNAelementsbyregulatoryproteinsformsthecentralmechanismofpromoter-specificregu-lation.However,ineukaryotes,particularlyinmammals,simplesite-specificbindingisinsufficienttogoverntheregulatoryprogrammesgiventhelargesizesandthecomplexityoftheirgenomes.TheorganizationofthesegenomesintocomplexnucleoproteinstructureswasoriginallythoughttobeonlyapackagingmechanismforDNA.Itisnowclearthatchromatinprovidesmarkedlyrestrictedaccessoftranscriptionfactorstoregulatorysitesinahighlycell-specificmanner1–4.Ascellsreplicateduringdifferentiation,therangeofelementsthatareavailableforbindingbyregulatoryproteinsconstantlychanges.Thisvariableaccesstoreg-ulatoryelementsisnowrecognizedtohaveakeyroleinnormalcelldevelopment,aswellasinalteredexpressionprofilesthatareassociatedwithmanydiseasestates5–8.AcentralquestionbecomeshowDNA-recognitionproteinsinteractwiththecellularenzymesthatactonchromatintoeitherdemarcateelementsforactionorsilencetheseelementsinagivencellularcontext.Althoughchromatintransitionscanoccuratmanylev-elsofbiologicalorganization,theaspectsofchromatinstructurethatimpingeonepigeneticregulationcanbedividedintothreegeneralareas.First,specifichistonemodificationshavebeenwidelystudied,andthereareclearsubsetsofhistonemarksthatareassociatedwithalteredactivitystatesforbothpromotersandenhancers.Second,aschromatinstructuresinhibitaccesstotheunderlyingDNAsequence,selectivelocalizedaccesstoregulatoryelements(thatis,‘open’chromatin)hasemergedasacommonfeatureofactiveregions9,10.Third,long-rangeinteractionsbetweenenhancersandtargetsoccuronawidescale;thechromosomeconfor-mationcapturemethodologi...