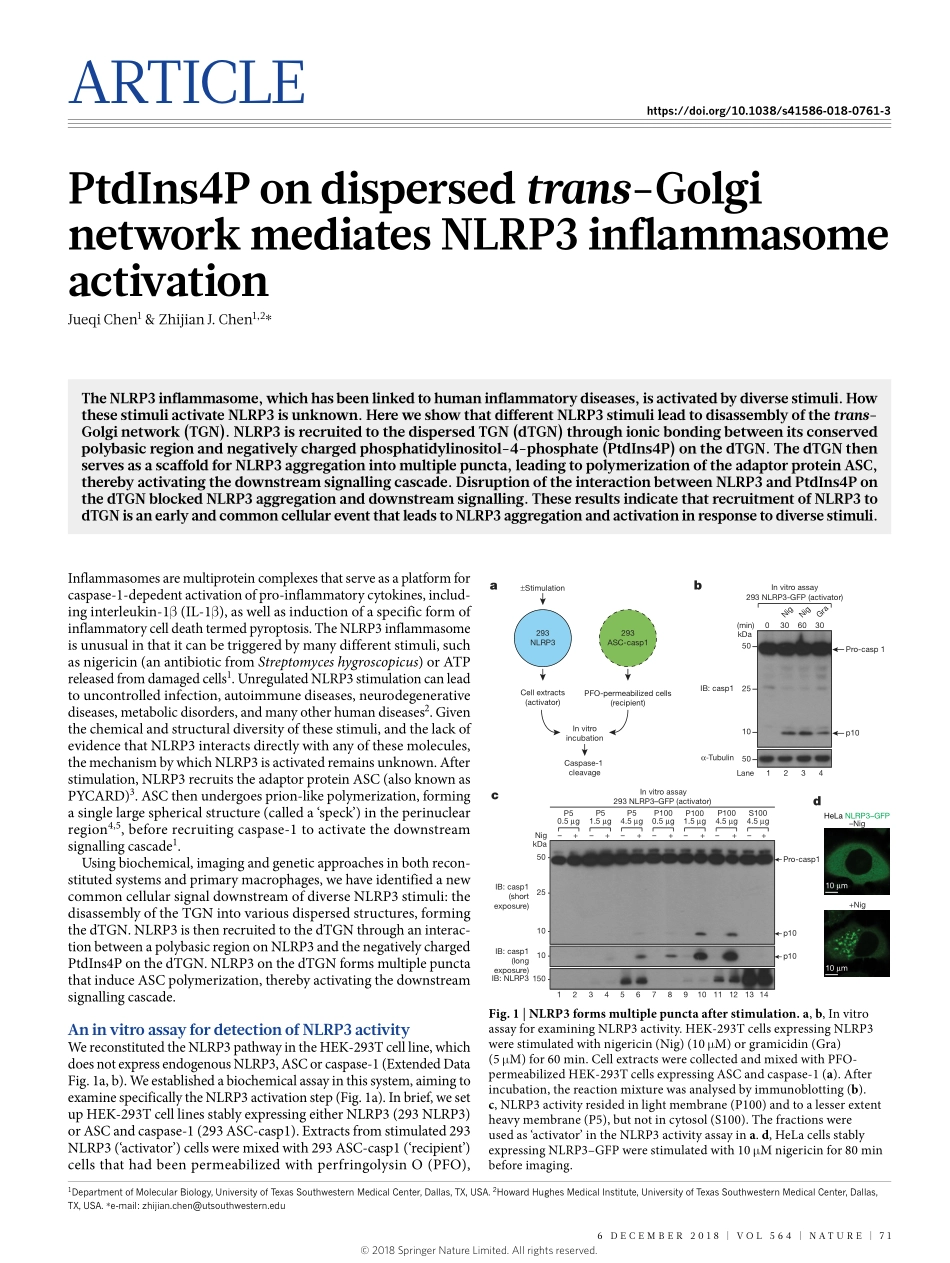
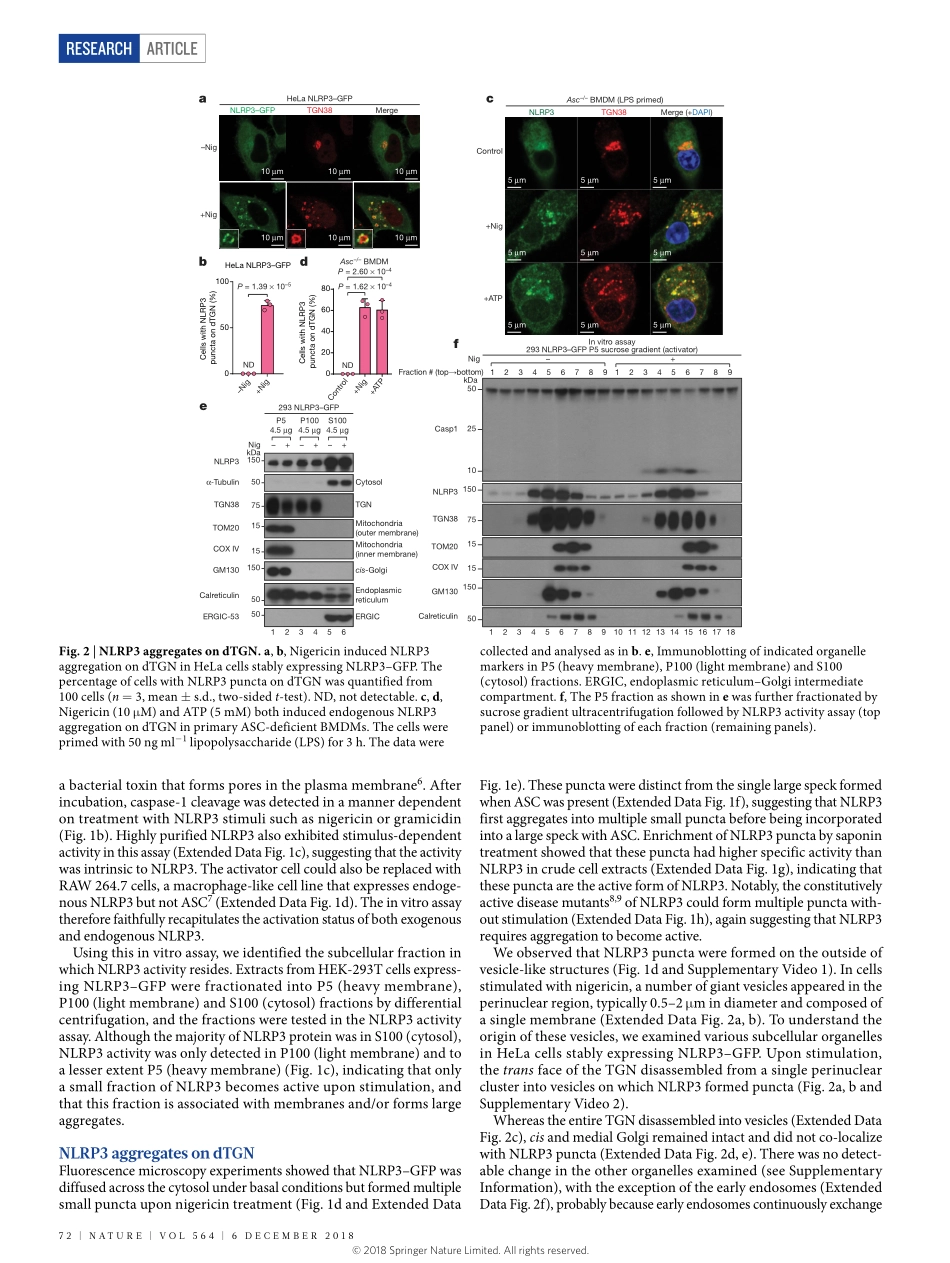
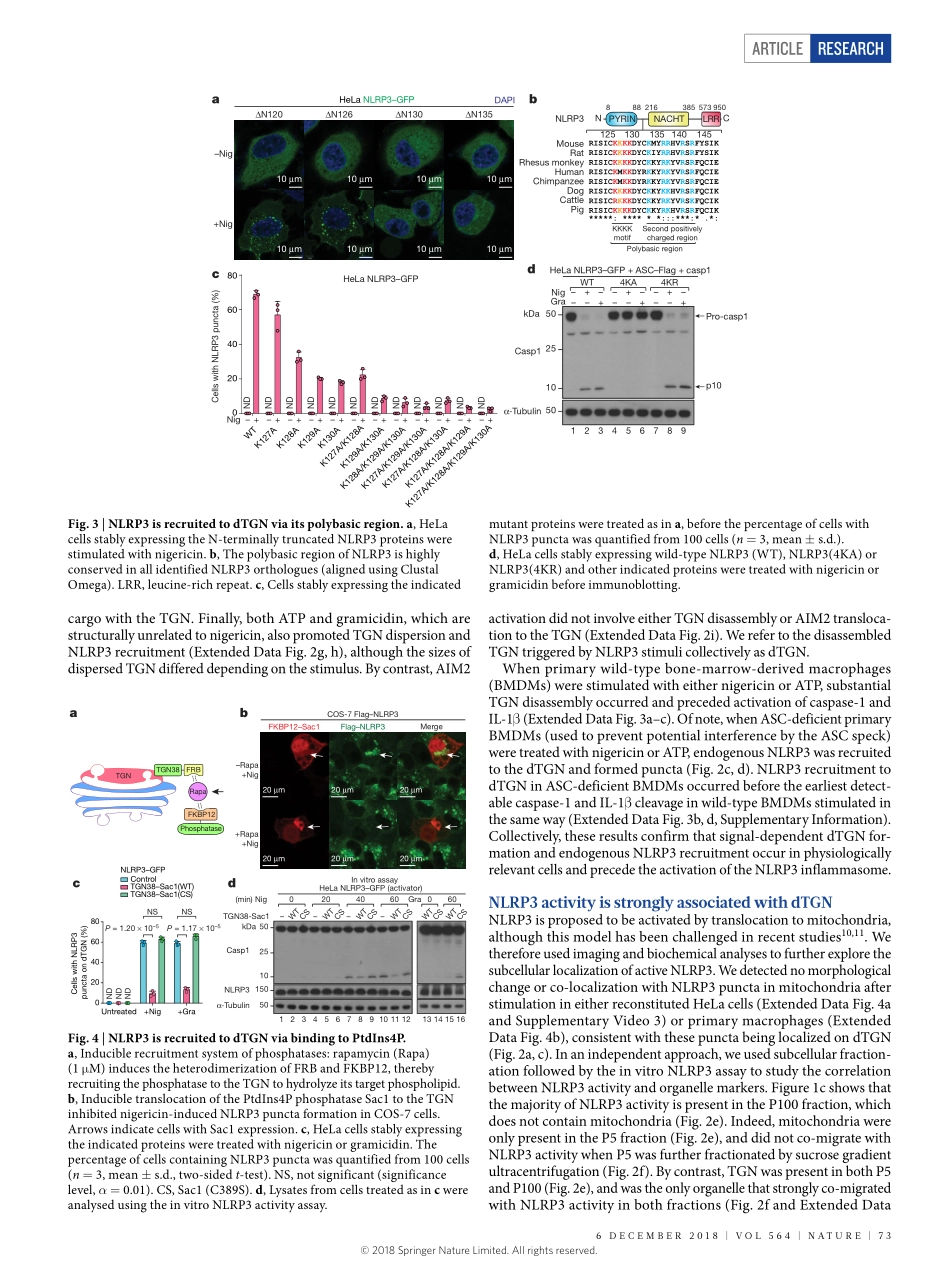
Articlehttps://doi.org/10.1038/s41586-018-0761-3PtdIns4Pondispersedtrans-GolginetworkmediatesNLRP3inflammasomeactivationJueqichen1&ZhijianJ.chen1,2*TheNLRP3inflammasome,whichhasbeenlinkedtohumaninflammatorydiseases,isactivatedbydiversestimuli.HowthesestimuliactivateNLRP3isunknown.HereweshowthatdifferentNLRP3stimulileadtodisassemblyofthetrans-Golginetwork(TGN).NLRP3isrecruitedtothedispersedTGN(dTGN)throughionicbondingbetweenitsconservedpolybasicregionandnegativelychargedphosphatidylinositol-4-phosphate(PtdIns4P)onthedTGN.ThedTGNthenservesasascaffoldforNLRP3aggregationintomultiplepuncta,leadingtopolymerizationoftheadaptorproteinASC,therebyactivatingthedownstreamsignallingcascade.DisruptionoftheinteractionbetweenNLRP3andPtdIns4PonthedTGNblockedNLRP3aggregationanddownstreamsignalling.TheseresultsindicatethatrecruitmentofNLRP3todTGNisanearlyandcommoncellulareventthatleadstoNLRP3aggregationandactivationinresponsetodiversestimuli.Inflammasomesaremultiproteincomplexesthatserveasaplatformforcaspase-1-depedentactivationofpro-inflammatorycytokines,includ-inginterleukin-1β(IL-1β),aswellasinductionofaspecificformofinflammatorycelldeathtermedpyroptosis.TheNLRP3inflammasomeisunusualinthatitcanbetriggeredbymanydifferentstimuli,suchasnigericin(anantibioticfromStreptomyceshygroscopicus)orATPreleasedfromdamagedcells1.UnregulatedNLRP3stimulationcanleadtouncontrolledinfection,autoimmunediseases,neurodegenerativediseases,metabolicdisorders,andmanyotherhumandiseases2.Giventhechemicalandstructuraldiversityofthesestimuli,andthelackofevidencethatNLRP3interactsdirectlywithanyofthesemolecules,themechanismbywhichNLRP3isactivatedremainsunknown.Afterstimulation,NLRP3recruitstheadaptorproteinASC(alsoknownasPYCARD)3.ASCthenundergoesprion-likepolymerization,formingasinglelargesphericalstructure(calleda‘speck’)intheperinuclearregion4,5,beforerecruitingcaspase-1toactivatethedownstreamsignallingcascade1.Usingbiochemical,imagingandgeneticapproachesinbothrecon-stitutedsystemsandprimarymacrophages,...