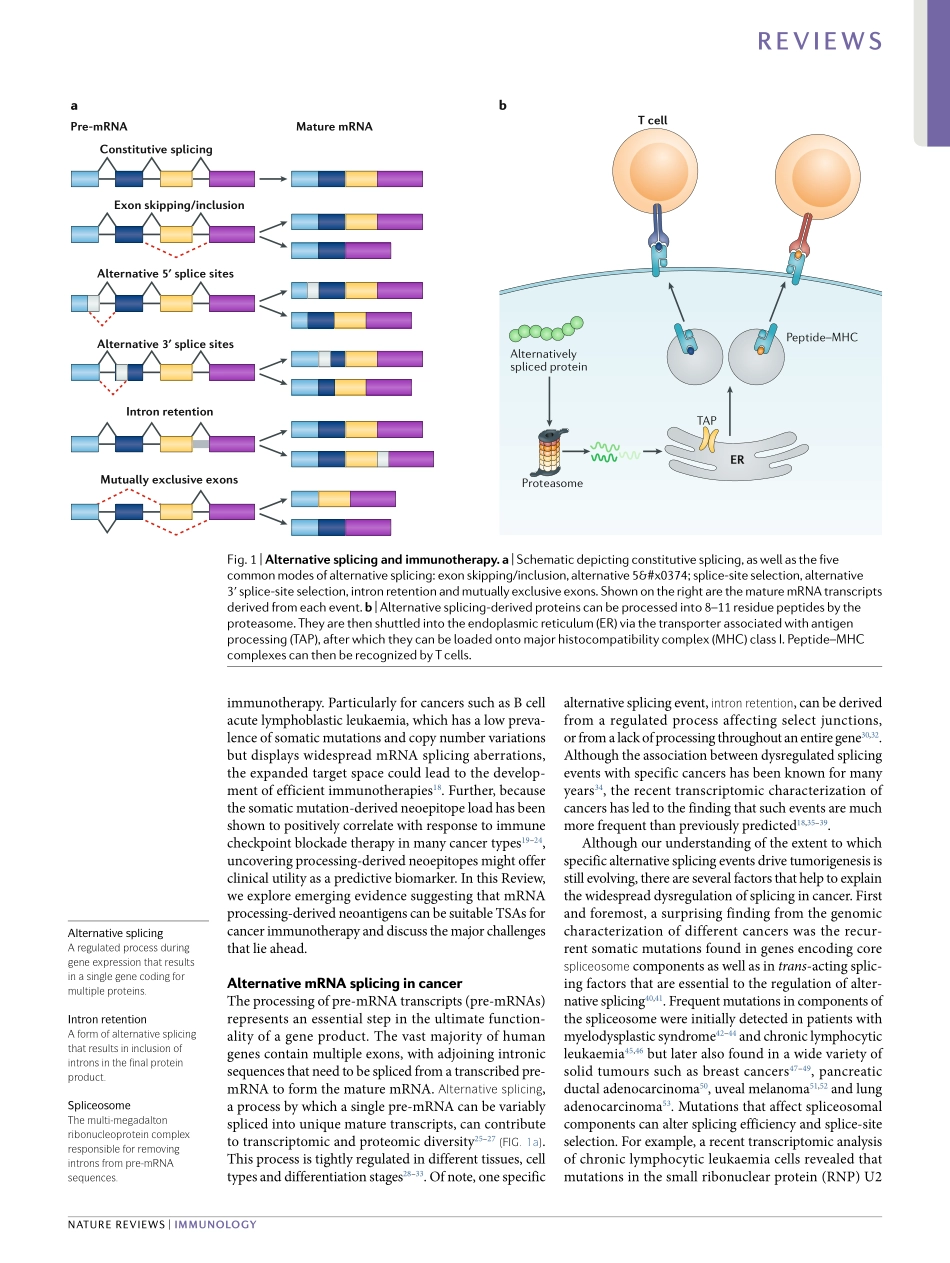
Theadventofimmunotherapyhasrevolutionizedthetreatmentofmanyformsofcancer.Itisnowwellestab-lishedthatTcellshavetheabilitytorejecttumoursuponbindingtoantigenicpeptides,derivedfromendogenouscellularproteinsorexogenousviralproteins,presentedbythemajorhistocompatibilitycomplex(MHC)onthesurfaceoftumourcells.Severalpromisingimmuno-therapeuticanticancerapproaches,suchastherapeuticvaccinesandTcellreceptorengineeredTcells(TCR-Tcells)foradoptivecelltherapy,relyontheidentificationofsuitabletargetantigens1.Historically,thefocushasbeenonthreeclassesoftumourantigens:tumour-specificsomaticnon-synonymousmutation-derivedneoantigens;cancergermlineantigens;andantigensderivedfromviralgenesthatareexpressedbyvirallyinfectedtumourcells(forexample,E6/E7fromhumanpapillomavirus)2.ClinicalstudieshaverevealedremarkableoutcomesbothforTCR-Tcelltherapytargetingcancergermlineanti-gensandforneoantigen-basedvaccines1.Forinstance,TCR-TcellstargetingNY-ESO-1,atumour-specificsharedgermlineantigen,havebeenshowntomediatesustainedantigen-specificantitumoureffectsinpatientswithmultiplemyeloma,aswellasseveralothercan-certypes3–5.Further,personalizedvaccinestargetingmutation-derivedneoantigenshavebeenshowntoelicitstrongneoepitope-specificTcellresponsesinpatientswithmelanoma(animmunologically‘hot’tumourwithahightumourmutationalburden(TMB))andglioblas-toma(animmunologically‘cold’tumourwitharelativelylowTMB)6–10.Despitetheunprecedenteddurableresponseratesobservedwithcancerimmunotherapiesinsomepatients,oneofthemajorobstaclesforthebroaderappli-cabilityofsuchtherapiesisthelackofcurrentlyknowntargetabletumour-specificantigens(TSAs)formanycancertypes1.Theselectionofappropriateantigensiscriticaltoensurethesafetyandefficacyofimmuno-therapy.Melanoma-associatedantigen3(MAGE-A3)andmelanomaantigenrecognizedbyTcells1(MART-1)havebeentwoleadingtargetantigensforTCR-Tcell-basedcancertherapiesduetotheirfrequentexpressioninseveraltumourtypesandtheirrestricted/lowexpres-sioninnormaltissues.However,severalclinicalcaseswith...