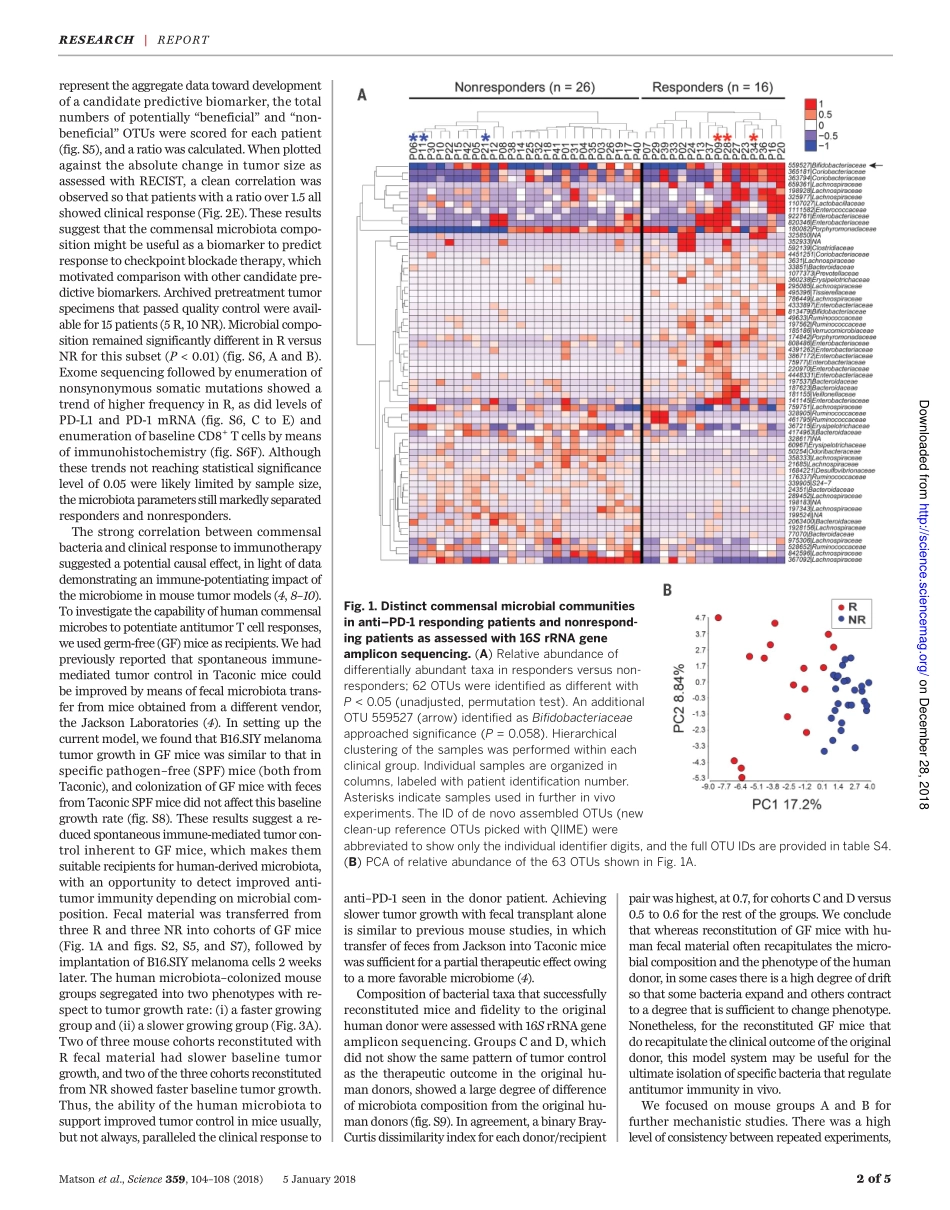
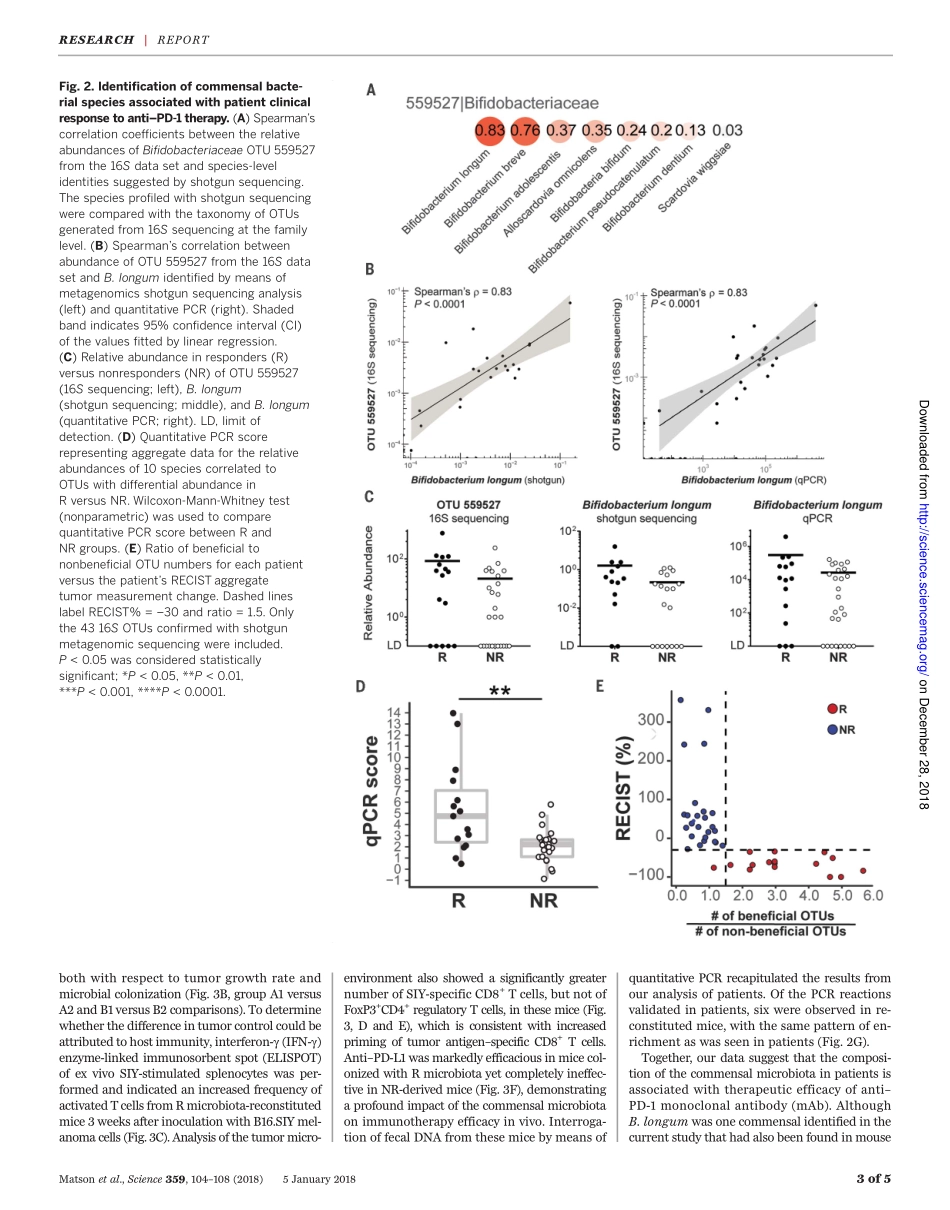
CANCERIMMUNOTHERAPYThecommensalmicrobiomeisassociatedwithanti–PD-1efficacyinmetastaticmelanomapatientsVyaraMatson,1*JessicaFessler,1*RiyueBao,2,3*TaraChongsuwat,4YuanyuanZha,4Maria-LuisaAlegre,4JasonJ.Luke,4ThomasF.Gajewski1,4†Anti–PD-1–basedimmunotherapyhashadamajorimpactoncancertreatmentbuthasonlybenefitedasubsetofpatients.Amongthevariablesthatcouldcontributetointerpatientheterogeneityisdifferentialcompositionofthepatients’microbiome,whichhasbeenshowntoaffectantitumorimmunityandimmunotherapyefficacyinpreclinicalmousemodels.Weanalyzedbaselinestoolsamplesfrommetastaticmelanomapatientsbeforeimmunotherapytreatment,throughanintegrationof16SribosomalRNAgenesequencing,metagenomicshotgunsequencing,andquantitativepolymerasechainreactionforselectedbacteria.Asignificantassociationwasobservedbetweencommensalmicrobialcompositionandclinicalresponse.BacterialspeciesmoreabundantinrespondersincludedBifidobacteriumlongum,Collinsellaaerofaciens,andEnterococcusfaecium.Reconstitutionofgerm-freemicewithfecalmaterialfromrespondingpatientscouldleadtoimprovedtumorcontrol,augmentedTcellresponses,andgreaterefficacyofanti–PD-L1therapy.Ourresultssuggestthatthecommensalmicrobiomemayhaveamechanisticimpactonantitumorimmunityinhumancancerpatients.Thetherapeuticefficacyofimmunothera-piestargetingthePD-1/PD-L1interactionisfavoredinpatientswhoshowevidenceofaTcell–inflamedtumormicroenvironmentatbaseline(1,2).Therefore,hostandtumorfactorsthatregulatethemagnitudeofendogenousimmuneprimingandTcellinfiltrationintothetumormicroenvironmentarebeingsoughtasanopportunitytofurtherexpandtherapeuticefficacy(3).Preclinicalstudieshaveindicatedthatthecompositionofthecommensalmicrobiomecouldexertamajorinfluence;micewithfavorablemi-crobiotashowedfargreatertherapeuticactivityofanti–PD-L1treatmentthandidmicewithanunfavorablemicrobiome,andthisbenefitcouldbetransferredbycohousingorfecaltransplant(4).Theseobservationspromptedananalogousanalysisofthehumanmicrobiomewithrespecttotherapeuticefficacyofanti–PD-1incan...