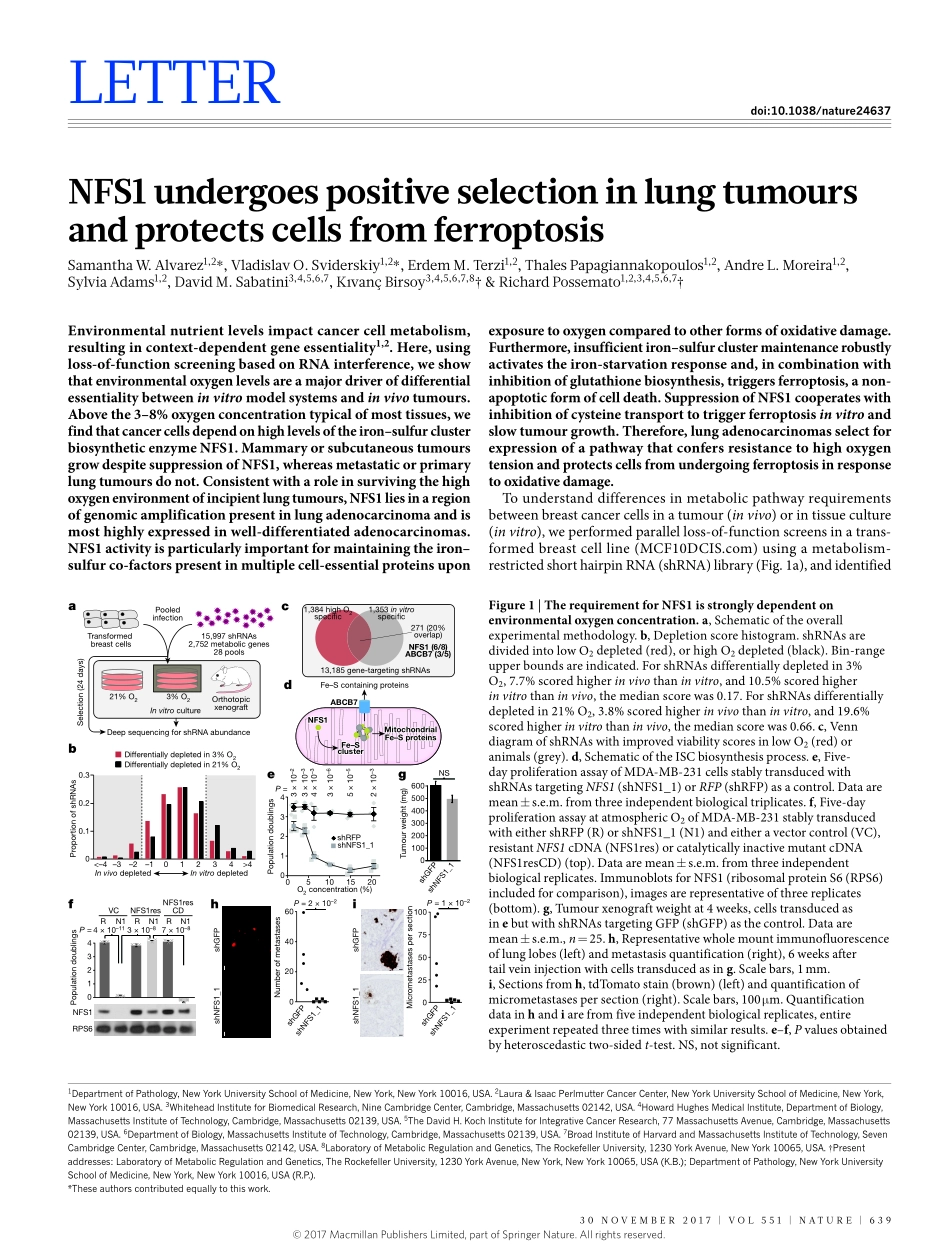
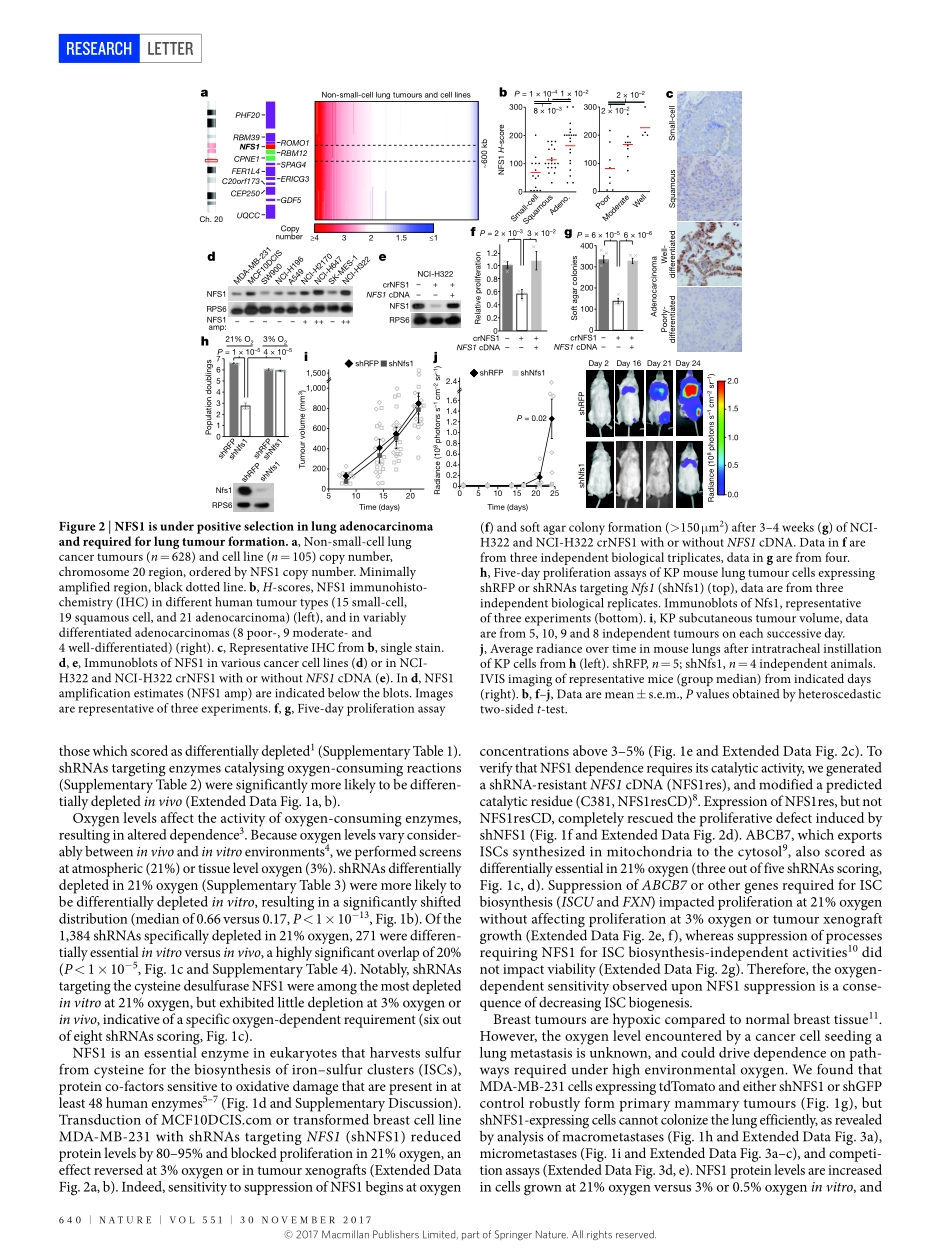
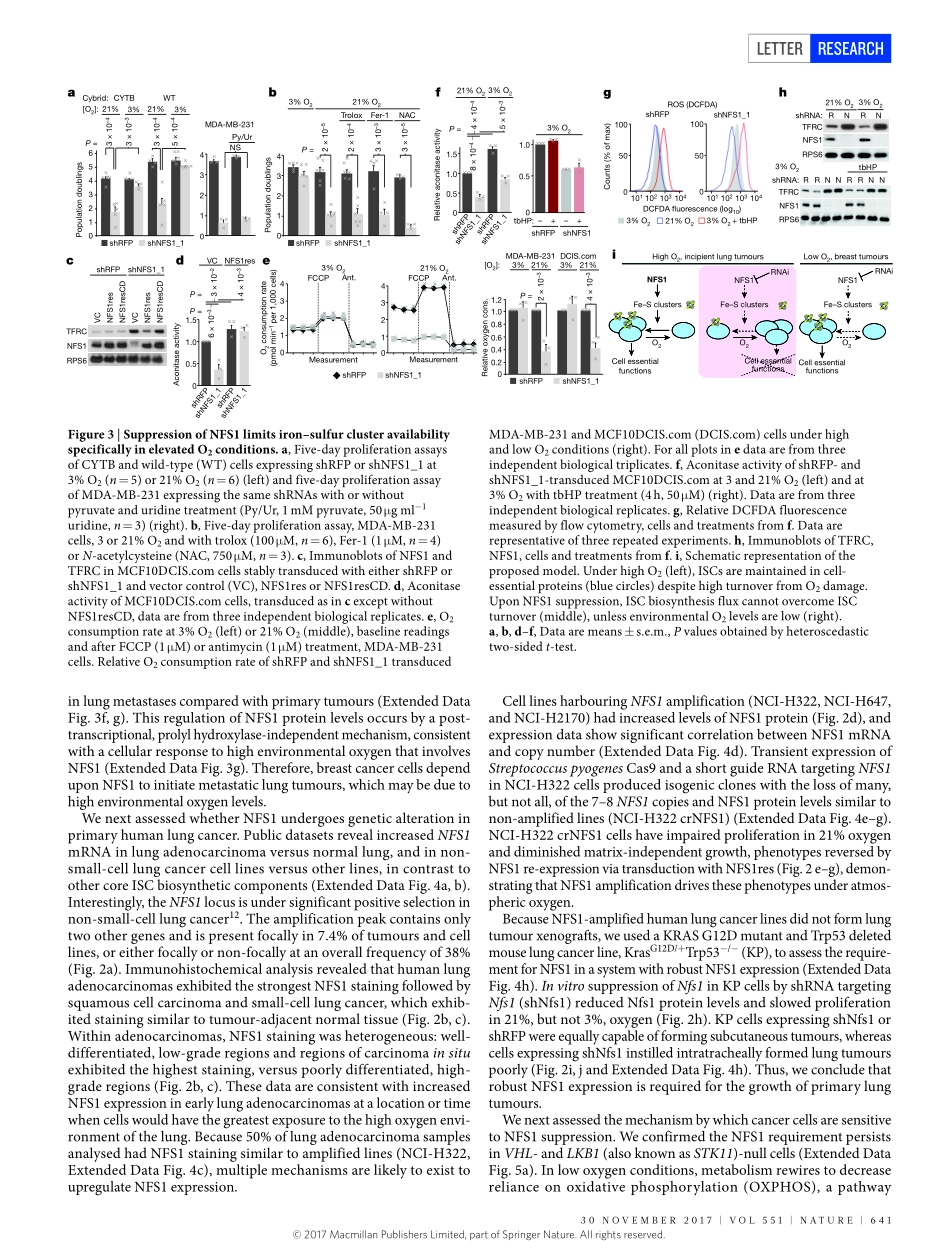
30november2017|voL551|nATUre|639LeTTerdoi:10.1038/nature24637NFS1undergoespositiveselectioninlungtumoursandprotectscellsfromferroptosisSamanthaW.Alvarez1,2*,vladislavo.Sviderskiy1,2*,erdemm.Terzi1,2,ThalesPapagiannakopoulos1,2,AndreL.moreira1,2,SylviaAdams1,2,Davidm.Sabatini3,4,5,6,7,Kıvançbirsoy3,4,5,6,7,8†&richardPossemato1,2,3,4,5,6,7†Environmentalnutrientlevelsimpactcancercellmetabolism,resultingincontext-dependentgeneessentiality1,2.Here,usingloss-of-functionscreeningbasedonRNAinterference,weshowthatenvironmentaloxygenlevelsareamajordriverofdifferentialessentialitybetweeninvitromodelsystemsandinvivotumours.Abovethe3–8%oxygenconcentrationtypicalofmosttissues,wefindthatcancercellsdependonhighlevelsoftheiron–sulfurclusterbiosyntheticenzymeNFS1.MammaryorsubcutaneoustumoursgrowdespitesuppressionofNFS1,whereasmetastaticorprimarylungtumoursdonot.Consistentwitharoleinsurvivingthehighoxygenenvironmentofincipientlungtumours,NFS1liesinaregionofgenomicamplificationpresentinlungadenocarcinomaandismosthighlyexpressedinwell-differentiatedadenocarcinomas.NFS1activityisparticularlyimportantformaintainingtheiron–sulfurco-factorspresentinmultiplecell-essentialproteinsuponexposuretooxygencomparedtootherformsofoxidativedamage.Furthermore,insufficientiron–sulfurclustermaintenancerobustlyactivatestheiron-starvationresponseand,incombinationwithinhibitionofglutathionebiosynthesis,triggersferroptosis,anon-apoptoticformofcelldeath.SuppressionofNFS1cooperateswithinhibitionofcysteinetransporttotriggerferroptosisinvitroandslowtumourgrowth.Therefore,lungadenocarcinomasselectforexpressionofapathwaythatconfersresistancetohighoxygentensionandprotectscellsfromundergoingferroptosisinresponsetooxidativedamage.Tounderstanddifferencesinmetabolicpathwayrequirementsbetweenbreastcancercellsinatumour(invivo)orintissueculture(invitro),weperformedparallelloss-of-functionscreensinatrans-formedbreastcellline(MCF10DCIS.com)usingametabolism-restrictedshorthairpinRNA(shRNA)library(Fig.1a),andidentif...