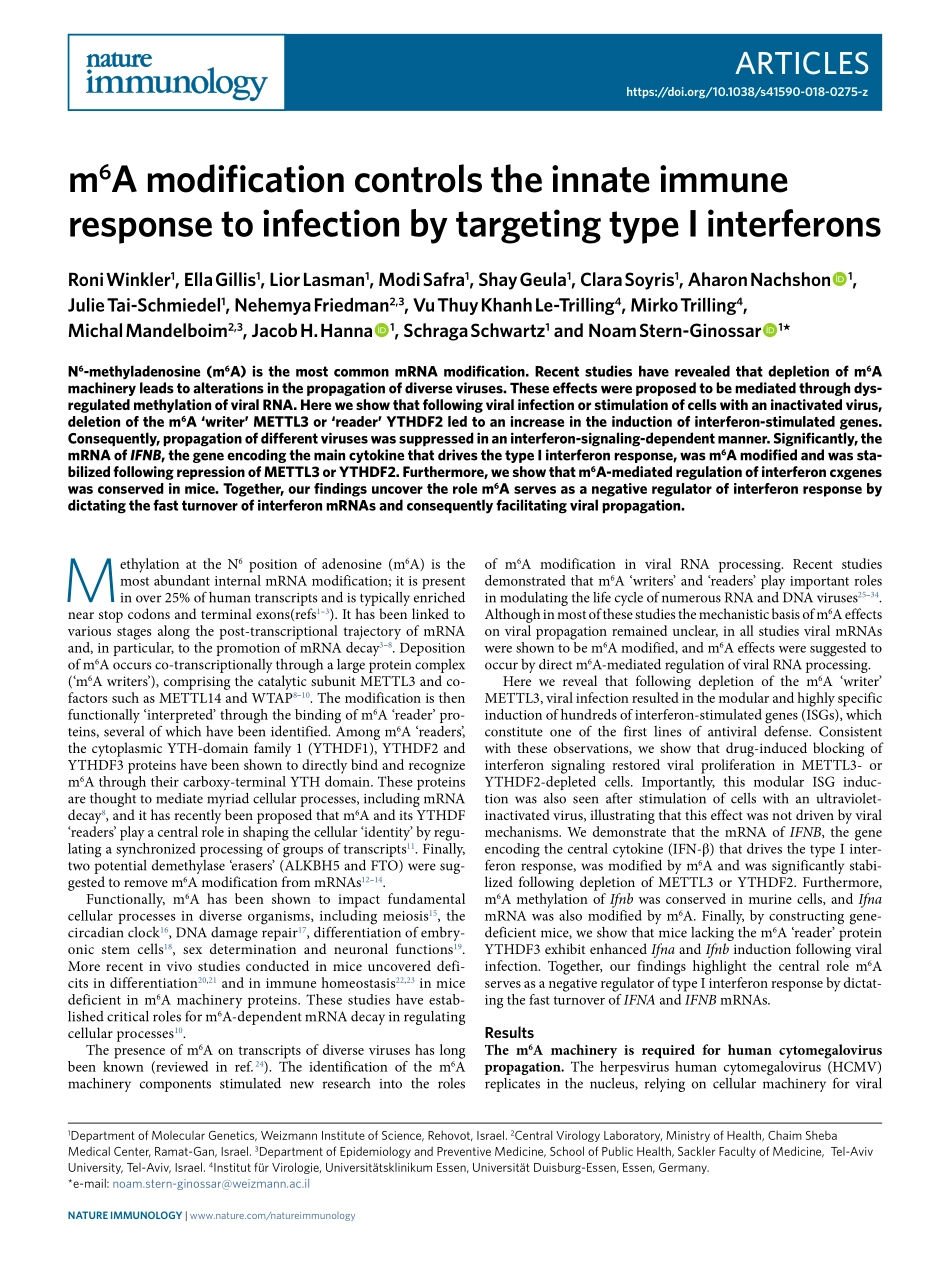
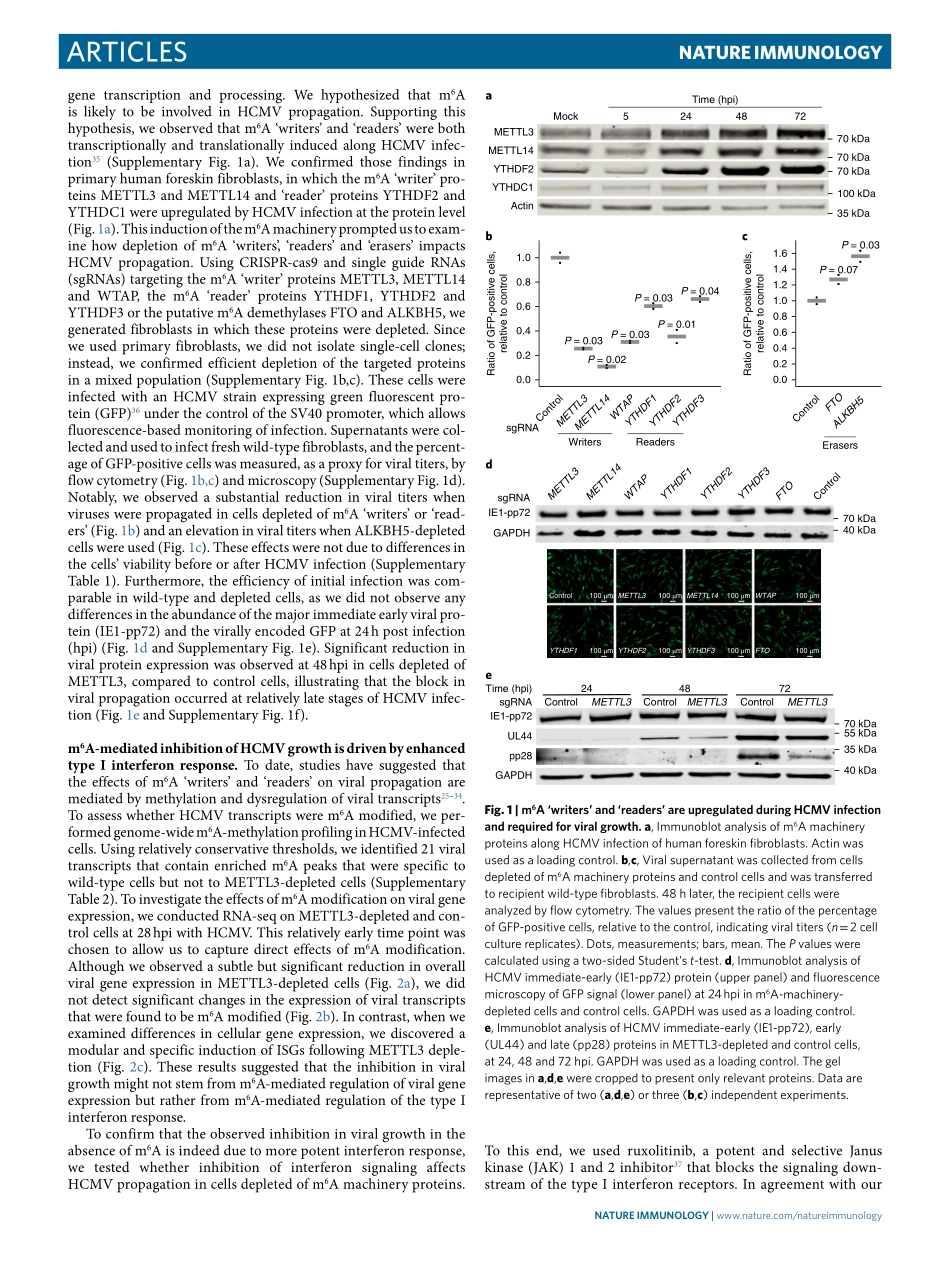
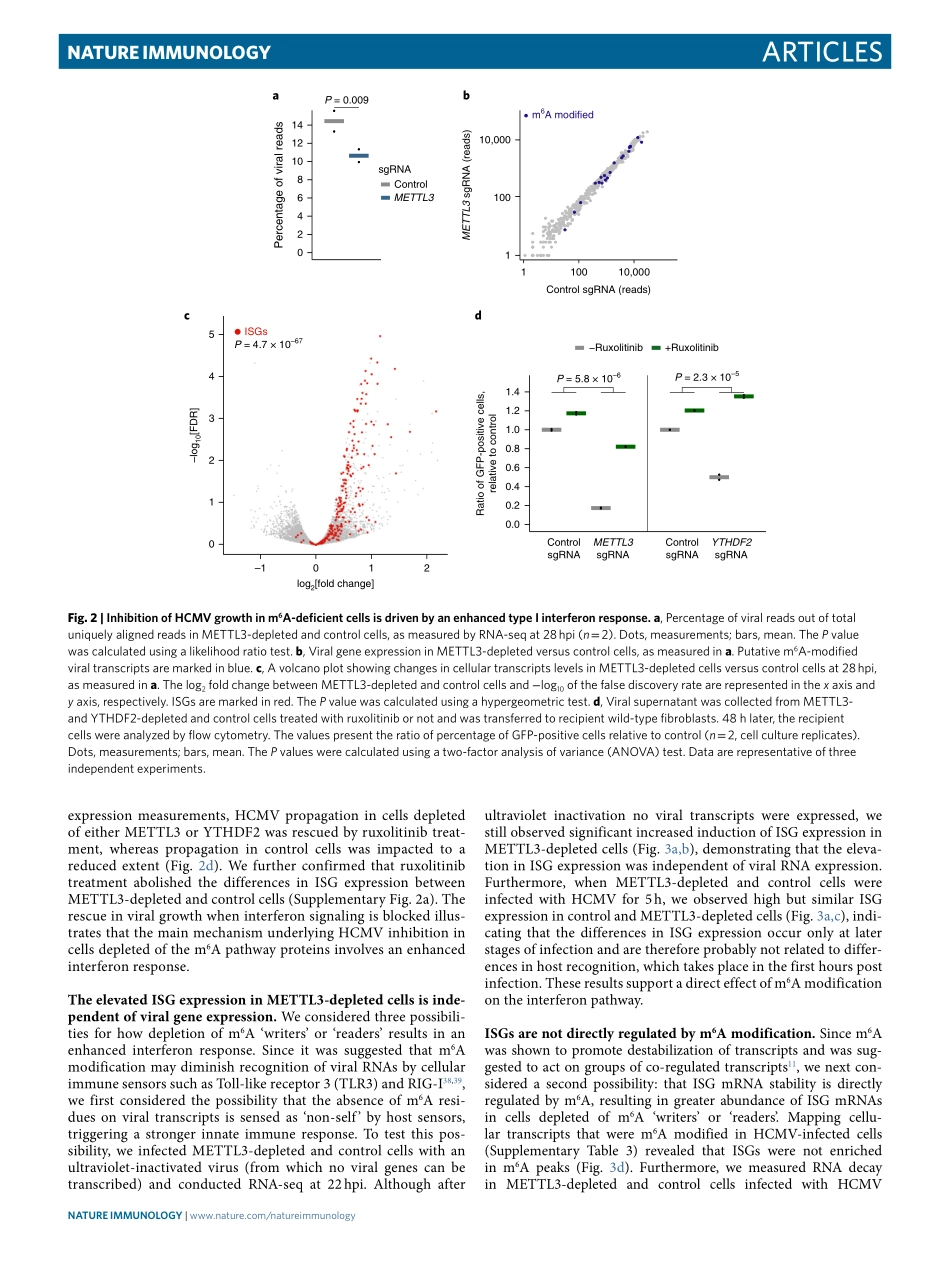
Articleshttps://doi.org/10.1038/s41590-018-0275-z1DepartmentofMolecularGenetics,WeizmannInstituteofScience,Rehovot,Israel.2CentralVirologyLaboratory,MinistryofHealth,ChaimShebaMedicalCenter,Ramat-Gan,Israel.3DepartmentofEpidemiologyandPreventiveMedicine,SchoolofPublicHealth,SacklerFacultyofMedicine,Tel-AvivUniversity,Tel-Aviv,Israel.4InstitutfürVirologie,UniversitätsklinikumEssen,UniversitätDuisburg-Essen,Essen,Germany.*e-mail:noam.stern-ginossar@weizmann.ac.ilMethylationattheN6positionofadenosine(m6A)isthemostabundantinternalmRNAmodification;itispresentinover25%ofhumantranscriptsandistypicallyenrichednearstopcodonsandterminalexons(refs1–3).Ithasbeenlinkedtovariousstagesalongthepost-transcriptionaltrajectoryofmRNAand,inparticular,tothepromotionofmRNAdecay3–8.Depositionofm6Aoccursco-transcriptionallythroughalargeproteincomplex(‘m6Awriters’),comprisingthecatalyticsubunitMETTL3andco-factorssuchasMETTL14andWTAP8–10.Themodificationisthenfunctionally‘interpreted’throughthebindingofm6A‘reader’pro-teins,severalofwhichhavebeenidentified.Amongm6A‘readers’,thecytoplasmicYTH-domainfamily1(YTHDF1),YTHDF2andYTHDF3proteinshavebeenshowntodirectlybindandrecognizem6Athroughtheircarboxy-terminalYTHdomain.Theseproteinsarethoughttomediatemyriadcellularprocesses,includingmRNAdecay8,andithasrecentlybeenproposedthatm6AanditsYTHDF‘readers’playacentralroleinshapingthecellular‘identity’byregu-latingasynchronizedprocessingofgroupsoftranscripts11.Finally,twopotentialdemethylase‘erasers’(ALKBH5andFTO)weresug-gestedtoremovem6AmodificationfrommRNAs12–14.Functionally,m6Ahasbeenshowntoimpactfundamentalcellularprocessesindiverseorganisms,includingmeiosis15,thecircadianclock16,DNAdamagerepair17,differentiationofembry-onicstemcells18,sexdeterminationandneuronalfunctions19.Morerecentinvivostudiesconductedinmiceuncovereddefi-citsindifferentiation20,21andinimmunehomeostasis22,23inmicedeficientinm6Amachineryproteins.Thesestudieshaveestab-lishedcriticalrolesform6A-dependentmRNAdecayi...