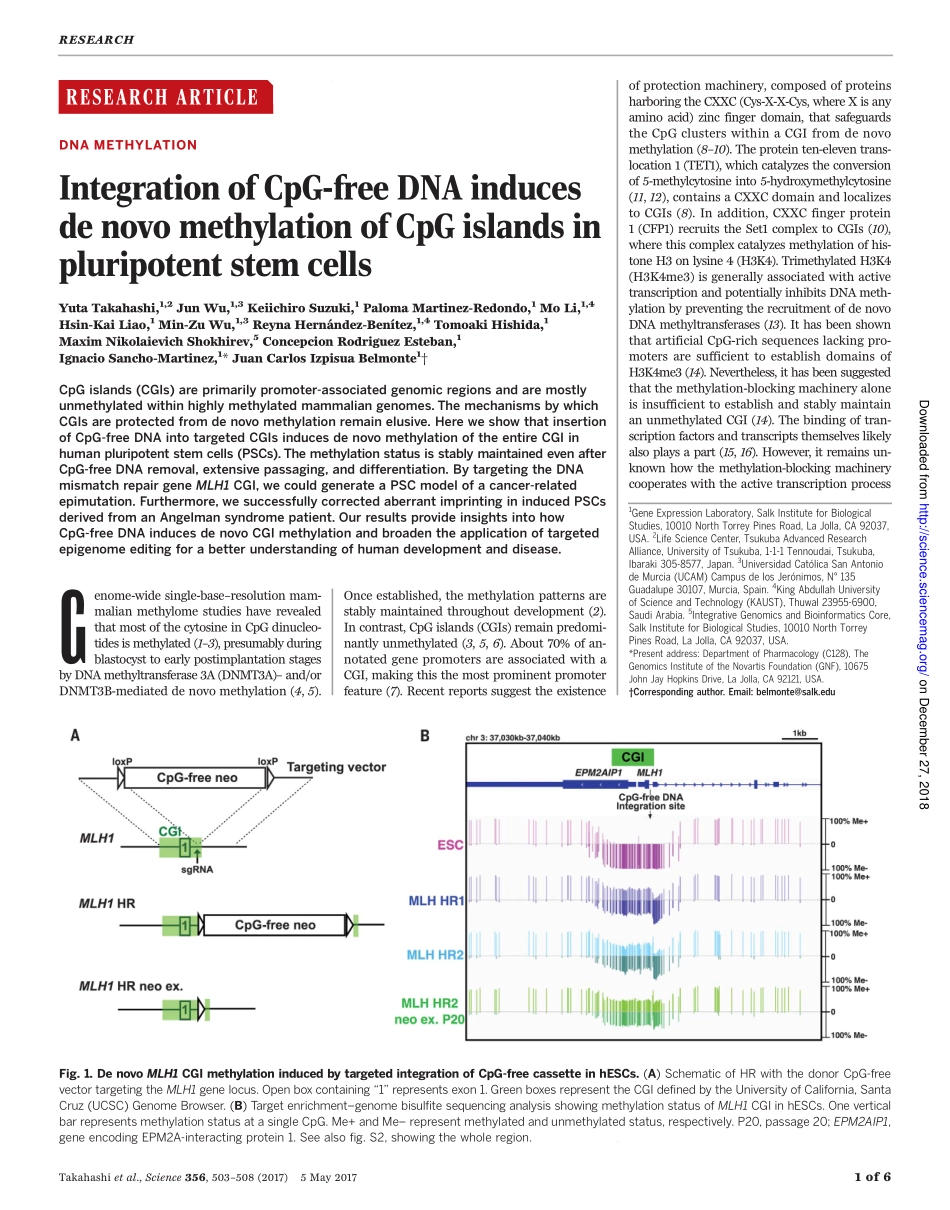
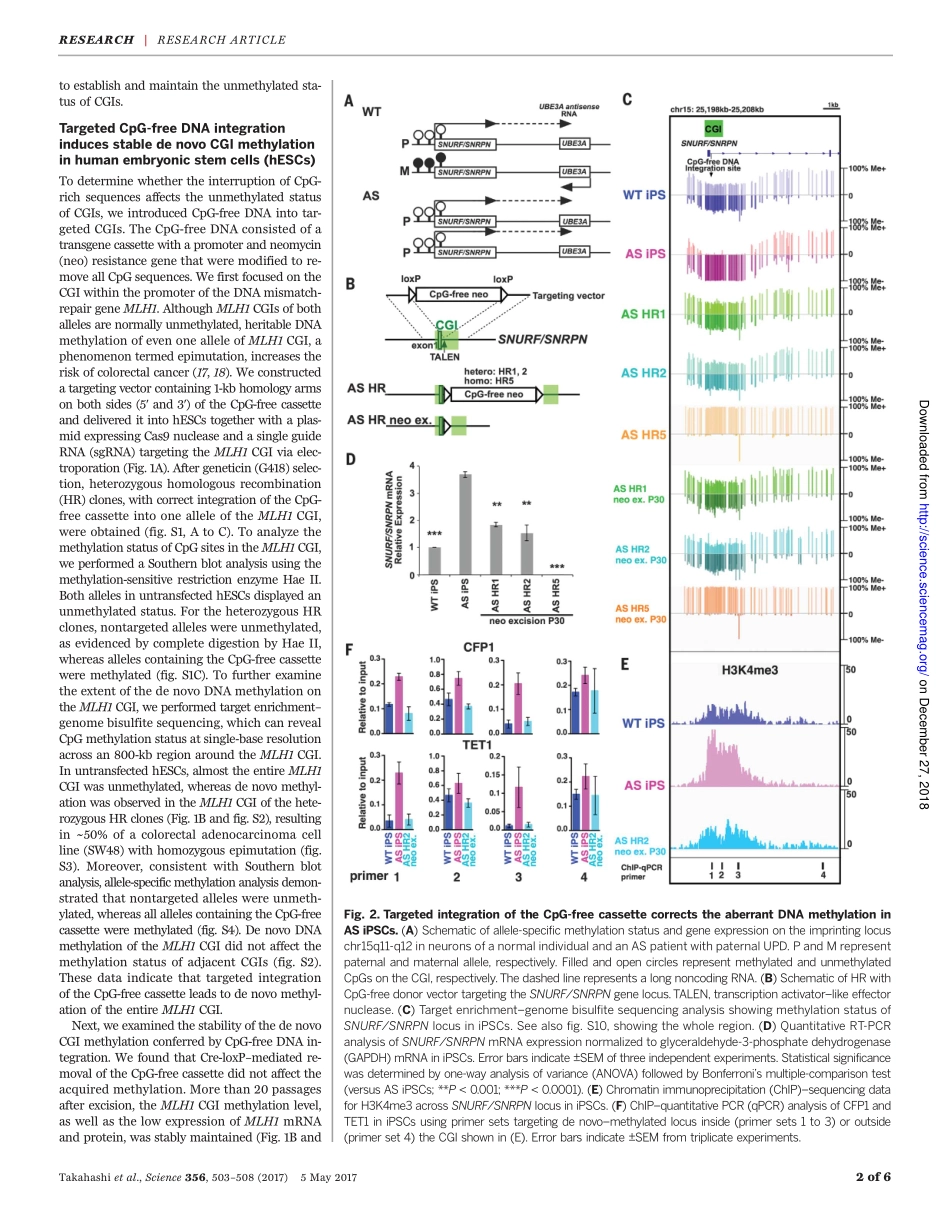
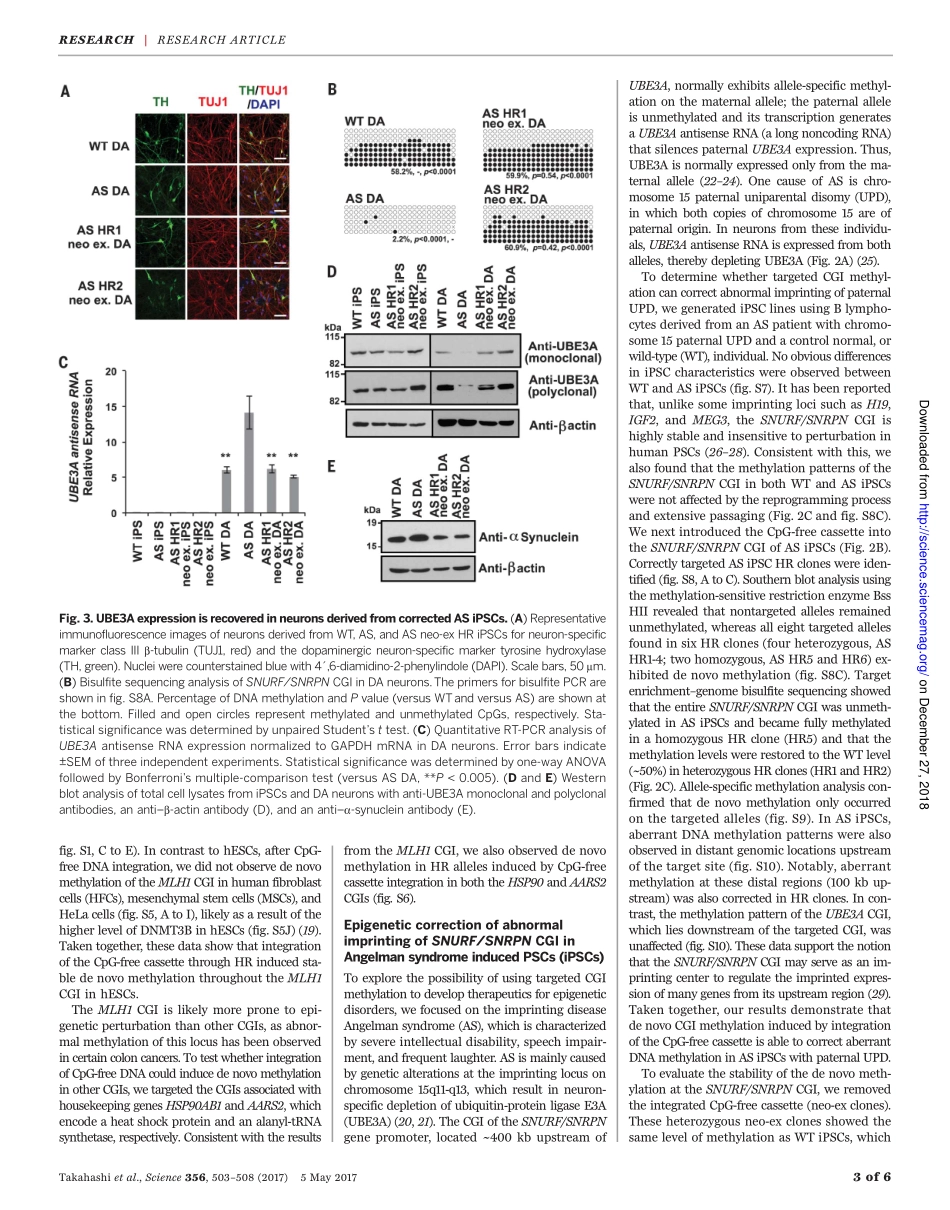
RESEARCHARTICLE◥DNAMETHYLATIONIntegrationofCpG-freeDNAinducesdenovomethylationofCpGislandsinpluripotentstemcellsYutaTakahashi,1,2JunWu,1,3KeiichiroSuzuki,1PalomaMartinez-Redondo,1MoLi,1,4Hsin-KaiLiao,1Min-ZuWu,1,3ReynaHernández-Benítez,1,4TomoakiHishida,1MaximNikolaievichShokhirev,5ConcepcionRodriguezEsteban,1IgnacioSancho-Martinez,1*JuanCarlosIzpisuaBelmonte1†CpGislands(CGIs)areprimarilypromoter-associatedgenomicregionsandaremostlyunmethylatedwithinhighlymethylatedmammaliangenomes.ThemechanismsbywhichCGIsareprotectedfromdenovomethylationremainelusive.HereweshowthatinsertionofCpG-freeDNAintotargetedCGIsinducesdenovomethylationoftheentireCGIinhumanpluripotentstemcells(PSCs).ThemethylationstatusisstablymaintainedevenafterCpG-freeDNAremoval,extensivepassaging,anddifferentiation.BytargetingtheDNAmismatchrepairgeneMLH1CGI,wecouldgenerateaPSCmodelofacancer-relatedepimutation.Furthermore,wesuccessfullycorrectedaberrantimprintingininducedPSCsderivedfromanAngelmansyndromepatient.OurresultsprovideinsightsintohowCpG-freeDNAinducesdenovoCGImethylationandbroadentheapplicationoftargetedepigenomeeditingforabetterunderstandingofhumandevelopmentanddisease.Genome-widesingle-base–resolutionmam-malianmethylomestudieshaverevealedthatmostofthecytosineinCpGdinucleo-tidesismethylated(1–3),presumablyduringblastocysttoearlypostimplantationstagesbyDNAmethyltransferase3A(DNMT3A)–and/orDNMT3B-mediateddenovomethylation(4,5).Onceestablished,themethylationpatternsarestablymaintainedthroughoutdevelopment(2).Incontrast,CpGislands(CGIs)remainpredomi-nantlyunmethylated(3,5,6).About70%ofan-notatedgenepromotersareassociatedwithaCGI,makingthisthemostprominentpromoterfeature(7).Recentreportssuggesttheexistenceofprotectionmachinery,composedofproteinsharboringtheCXXC(Cys-X-X-Cys,whereXisanyaminoacid)zincfingerdomain,thatsafeguardstheCpGclusterswithinaCGIfromdenovomethylation(8–10).Theproteinten-eleventrans-location1(TET1),whichcatalyzestheconversionof5-methylcytosineinto5-hydroxymethylcytosine(11...