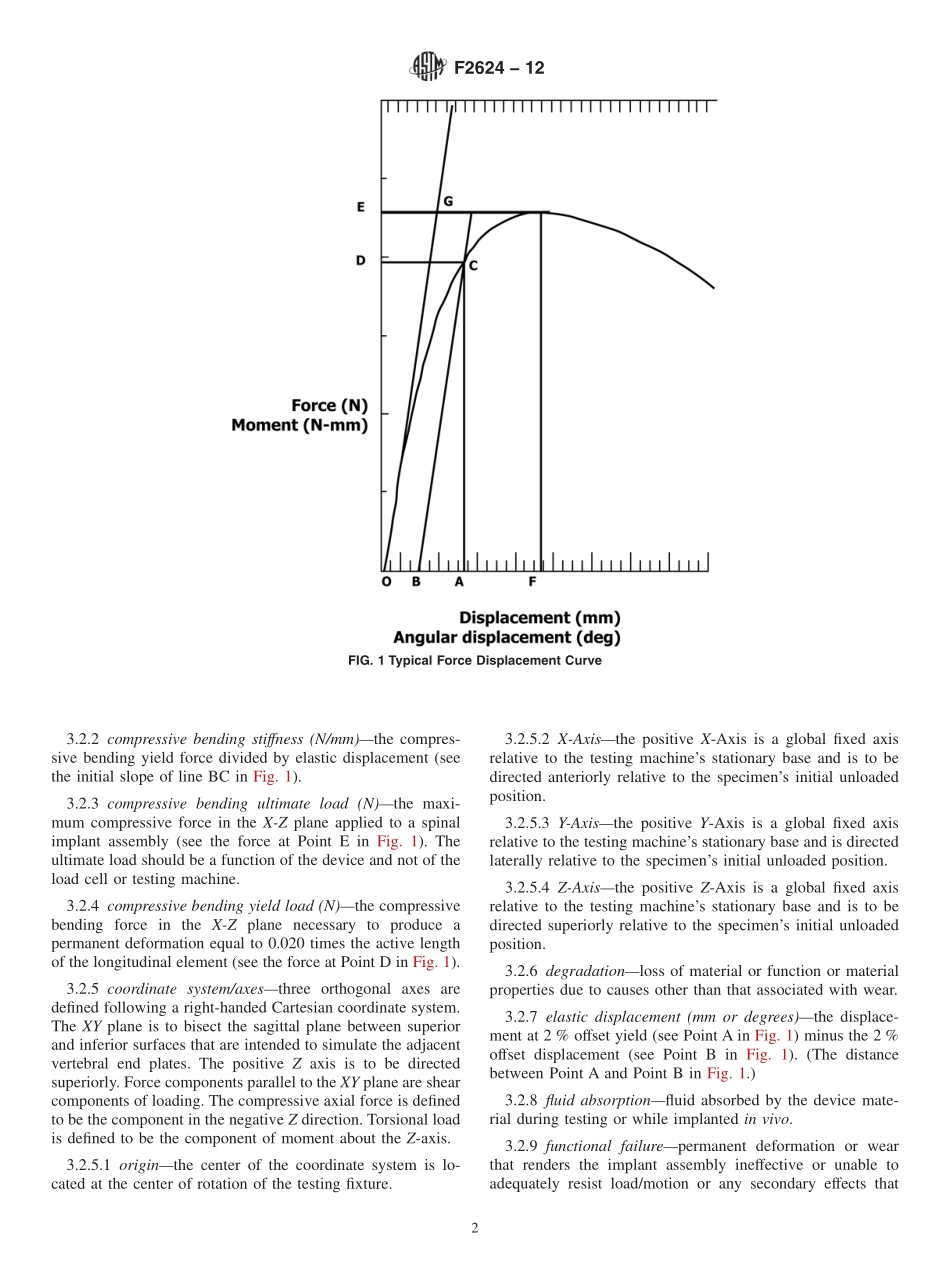
Designation:F2624−12StandardTestMethodforStatic,Dynamic,andWearAssessmentofExtra-DiscalSingleLevelSpinalConstructs1ThisstandardisissuedunderthefixeddesignationF2624;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginaladoptionor,inthecaseofrevision,theyearoflastrevision.Anumberinparenthesesindicatestheyearoflastreapproval.Asuperscriptepsilon(´)indicatesaneditorialchangesincethelastrevisionorreapproval.1.Scope1.1Thistestmethoddescribesmethodstoassessthestaticanddynamicpropertiesofsinglelevelspinalconstructs.1.2Anoptionforassessingwearusingaweightlossmethodandadimensionalanalysisisgiven.Thismethod,describedherein,isusedfortheanalysisofdevicesintendedformotionpreservation,usingtestingmediumasdefinedinthisstandard(6.1).1.3Thistestmethodisnotintendedtoaddressanypotentialfailuremodeasitrelatestothefixationofthedevicetoitsbonyinterfaces.1.4Itistheintentofthistestmethodtoenablesinglelevelextra-discalspinalconstructswithregardtokinematic,functional,andwearcharacteristicswhentestedunderthespecifiedconditions.1.5Thistestmethodisnotintendedtoaddressfacetarthroplastydevices.1.6Inorderthatthedatabereproducibleandcomparablewithinandbetweenlaboratories,itisessentialthatuniformproceduresbeestablished.Thistestmethodisintendedtofacilitateuniformtestingmethodsanddatareporting.1.7Themotionprofilesspecifiedbythistestmethoddonotnecessarilyaccuratelyreproducethoseoccurringinvivo.Ratherthismethodprovidesusefulboundary/endpointcondi-tionsforevaluatingimplantdesignsinafunctionalmanner.1.8Thistestmethodisnotintendedtobeaperformancestandard.Itistheresponsibilityoftheuserofthistestmethodtocharacterizethesafetyandeffectivenessofthedeviceunderevaluation.1.9Multipletestmethodsareincludedinthisstandard.However,itmustbenotedthattheuserisnotobligatedtotestusingallofthedescribedmethods.Instead,theusershouldonlyselecttestmethodsthatareappropriateforaparticulardevicedesign.Inmostinstances,onlyasubsetofthehereindescribedtestmethodswillberequired.1.10ThevaluesstatedinSIunitsaretoberega...